Efficacy of olaparib in advanced cancers with germline or somatic mutations in BRCA1, BRCA2, CHEK2 and ATM, a Belgian Precision tumor-agnostic phase II study
- PMID: 37852034
- PMCID: PMC10774963
- DOI: 10.1016/j.esmoop.2023.102041
Efficacy of olaparib in advanced cancers with germline or somatic mutations in BRCA1, BRCA2, CHEK2 and ATM, a Belgian Precision tumor-agnostic phase II study
Abstract
Background: The Belgian Precision initiative aims to maximize the implementation of tumor-agnostic next-generation sequencing in patients with advanced cancer and enhance access to molecularly guided treatment options. Academic tumor-agnostic basket phase II studies are part of this initiative. The current investigator-driven trial aimed to investigate the efficacy of olaparib in advanced cancers with a (likely) pathogenic mutation (germline or somatic) in a gene that plays a role in homologous recombination (HR).
Patients and methods: This open-label, multi-cohort, phase II study examines the efficacy of olaparib in patients with an HR gene mutation in their tumor and disease progression on standard of care. Patients with a somatic or germline mutation in the same gene define a cohort. For each cohort, a Simon minimax two-stage design was used. If a response was observed in the first 13 patients, 14 additional patients were included. Here, we report the results on four completed cohorts: patients with a BRCA1, BRCA2, CHEK2 or ATM mutation.
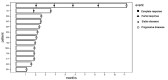
Results: The overall objective response rate across different tumor types was 11% in the BRCA1-mutated (n = 27) and 21% in the BRCA2-mutated (n = 27) cohorts. Partial responses were seen in pancreatic cancer, gallbladder cancer, endocrine carcinoma of the pancreas and parathyroid cancer. One patient with a BRCA2 germline-mutated colon cancer has an ongoing complete response with 19+ months on treatment. Median progression-free survival in responding patients was 14+ months (5-34+ months). The clinical benefit rate was 63% in the BRCA1-mutated and 46% in the BRCA2-mutated cohorts. No clinical activity was observed in the ATM (n = 13) and CHEK2 (n = 14) cohorts.
Conclusion: Olaparib showed efficacy in different cancer types harboring somatic or germline mutations in the BRCA1/2 genes but not in ATM and CHEK2. Patients with any cancer type harboring BRCA1/2 mutations should have access to olaparib.
Keywords: ATM; BRCA1; BRCA2; CHEK2; agnostic NGS; biliary tract cancer; colorectal cancer; olaparib; parathyroid cancer.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures



References
-
- Lopacinska-Joergensen J., Oliveira D., Poulsen T.S., Hoegdall C.K., Hoegdall E.V. Somatic variants in DNA damage response genes in ovarian cancer patients using whole-exome sequencing. Anticancer Res. 2023;43(5):1891–1900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

