CD36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression
- PMID: 37852255
- PMCID: PMC11748917
- DOI: 10.1016/j.cmet.2023.09.012
CD36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression
Abstract
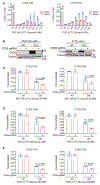
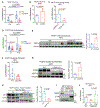
A high-fat diet (HFD) promotes metastasis through increased uptake of saturated fatty acids (SFAs). The fatty acid transporter CD36 has been implicated in this process, but a detailed understanding of CD36 function is lacking. During matrix detachment, endoplasmic reticulum (ER) stress reduces SCD1 protein, resulting in increased lipid saturation. Subsequently, CD36 is induced in a p38- and AMPK-dependent manner to promote preferential uptake of monounsaturated fatty acids (MUFAs), thereby maintaining a balance between SFAs and MUFAs. In attached cells, CD36 palmitoylation is required for MUFA uptake and protection from palmitate-induced lipotoxicity. In breast cancer mouse models, CD36-deficiency induced ER stress while diminishing the pro-metastatic effect of HFD, and only a palmitoylation-proficient CD36 rescued this effect. Finally, AMPK-deficient tumors have reduced CD36 expression and are metastatically impaired, but ectopic CD36 expression restores their metastatic potential. Our results suggest that, rather than facilitating HFD-driven tumorigenesis, CD36 plays a supportive role by preventing SFA-induced lipotoxicity.
Keywords: CD36; cancer metabolism; fatty acids; matrix detachment; metastasis; palmitoylation.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

