Clinical effectiveness of septoplasty versus medical management for nasal airways obstruction: multicentre, open label, randomised controlled trial
- PMID: 37852641
- PMCID: PMC10583133
- DOI: 10.1136/bmj-2023-075445
Clinical effectiveness of septoplasty versus medical management for nasal airways obstruction: multicentre, open label, randomised controlled trial
Abstract
Objective: To assess the clinical effectiveness of septoplasty.
Design: Multicentre, randomised controlled trial.
Setting: 17 otolaryngology clinics in the UK's National Health Service.
Participants: 378 adults (≥18 years, 67% men) newly referred with symptoms of nasal obstruction associated with septal deviation and at least moderate symptoms of nasal obstruction (score >30 on the Nasal Obstruction and Symptom Evaluation (NOSE) scale).
Interventions: Participants were randomised 1:1 to receive either septoplasty (n=188) or defined medical management (n=190, nasal steroid and saline spray for six months), stratified by baseline symptom severity and sex.
Main outcome measures: The primary outcome measure was patient reported score on the Sino-Nasal Outcome Test-22 (SNOT-22) at six months, with 9 points defined as the minimal clinically important difference. Secondary outcomes included quality of life and objective nasal airflow measures.
Results: Mean SNOT-22 scores at six months were 19.9 (95% confidence interval 17.0 to 22.7) in the septoplasty arm (n=152, intention-to-treat population) and 39.5 (36.1 to 42.9) in the medical management arm (n=155); an estimated 20.0 points lower (better) for participants randomised to receive septoplasty (95% confidence interval 16.4 to 23.6, P<0.001, adjusted for baseline continuous SNOT-22 score and the stratification variables sex and baseline NOSE severity categories). Greater improvement in SNOT-22 scores was predicted by higher baseline symptom severity scores. Quality of life outcomes and nasal airflow measures (including peak nasal inspiratory flow and absolute inhalational nasal partitioning ratio) improved more in participants in the septoplasty group. Readmission to hospital with bleeding after septoplasty occurred in seven participants (4% of 174 who had septoplasty), and a further 20 participants (12%) required antibiotics for infections.
Conclusions: Septoplasty is a more effective intervention than a defined medical management regimen with a nasal steroid and saline spray in adults with nasal obstruction associated with a deviated nasal septum.
Trial registration: ISRCTN Registry ISRCTN16168569.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institute for Health and Care Research and salaries part funded through host institutions (SC, JOH, TF, TH, NR, LR, AB, DDS, LT, KR, AJS, JD, MD, KL, CWilson, SK, JWilson, and MDT); no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
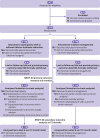


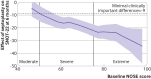
Comment in
-
Septoplasty for nasal obstruction.BMJ. 2023 Oct 18;383:2341. doi: 10.1136/bmj.p2341. BMJ. 2023. PMID: 37852637 No abstract available.
References
-
- NHS Digital Hospital Episode Statistics. Admitted Patient Care – England, 2019-2020. https://digital.nhs.uk/data-and-information/publications/statistical/hos... (accessed Jan 2020).
-
- Bedfordshire and Hertfordshire Priorities Forum Guidance Number. 71. Subject: Septoplasty, rhinoplasty, and septorhinoplasty. Bedfordshire and Hertfordshire Clinical Commissioning Groups. 2016. https://www.enhertsccg.nhs.uk/sites/default/files/documents/Sep2016/Guid...
-
- National Institute for Health and Care Research - Dissemination Centre. NIHR Evidence. Surgery for a deviated nasal septum improves quality of life more than non-surgical approaches. 2019. https://evidence.nihr.ac.uk/alert/surgery-for-a-deviated-nasal-septum-im...
-
- Rennie KJ, O’Hara J, Rousseau N, et al. NAIROS Study Group . Nasal Airway Obstruction Study (NAIROS): a phase III, open-label, mixed-methods, multicentre randomised controlled trial of septoplasty versus medical management of a septal deviation with nasal obstruction. Trials 2020;21:179. 10.1186/s13063-020-4081-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
