Rosuvastatin versus atorvastatin treatment in adults with coronary artery disease: secondary analysis of the randomised LODESTAR trial
- PMID: 37852649
- PMCID: PMC10583134
- DOI: 10.1136/bmj-2023-075837
Rosuvastatin versus atorvastatin treatment in adults with coronary artery disease: secondary analysis of the randomised LODESTAR trial
Abstract
Objective: To compare the long term efficacy and safety of rosuvastatin with atorvastatin treatment in adults with coronary artery disease.
Design: Randomised, open label, multicentre trial.
Setting: 12 hospitals in South Korea, September 2016 to November 2019.
Participants: 4400 adults (age ≥19 years) with coronary artery disease.
Interventions: Participants were assigned to receive either rosuvastatin (n=2204) or atorvastatin (n=2196) using 2×2 factorial randomisation.
Main outcome measures: The primary outcome was a three year composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation. Secondary outcomes were safety endpoints: new onset diabetes mellitus; hospital admissions due to heart failure; deep vein thrombosis or pulmonary thromboembolism; endovascular revascularisation for peripheral artery disease; aortic intervention or surgery; end stage kidney disease; discontinuation of study drugs owing to intolerance; cataract surgery; and a composite of laboratory detected abnormalities.
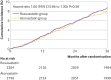
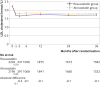
Results: 4341 of the 4400 participants (98.7%) completed the trial. Mean daily dose of study drugs was 17.1 mg (standard deviation (SD) 5.2 mg) in the rosuvastatin group and 36.0 (12.8) mg in the atorvastatin group at three years (P<0.001). The primary outcome occurred in 189 participants (8.7%) in the rosuvastatin group and 178 (8.2%) in the atorvastatin group (hazard ratio 1.06, 95% confidence interval 0.86 to 1.30; P=0.58). The mean low density lipoprotein (LDL) cholesterol level during treatment was 1.8 mmol/L (SD 0.5 mmol/L) in the rosuvastatin group and 1.9 (0.5) mmol/L in the atorvastatin group (P<0.001). The rosuvastatin group had a higher incidence of new onset diabetes mellitus requiring initiation of antidiabetics (7.2% v 5.3%; hazard ratio 1.39, 95% confidence interval 1.03 to 1.87; P=0.03) and cataract surgery (2.5% v 1.5%; 1.66, 1.07 to 2.58; P=0.02). Other safety endpoints did not differ between the two groups.
Conclusions: In adults with coronary artery disease, rosuvastatin and atorvastatin showed comparable efficacy for the composite outcome of all cause death, myocardial infarction, stroke, or any coronary revascularisation at three years. Rosuvastatin was associated with lower LDL cholesterol levels but a higher risk of new onset diabetes mellitus requiring antidiabetics and cataract surgery compared with atorvastatin.
Trial registration: ClinicalTrials.gov NCT02579499.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: the study was funded by a grant from Sam Jin Pharmaceutical and Chong Kun Dang Pharmaceutical. M-KH has received speaker’s fees from Medtronic, Edward Lifesciences, and Viatris Korea, and institutional research grants from Sam Jin Pharmaceutical and Chong Kun Dang Pharmaceutical; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:e285-350. 10.1016/j.jacc.2018.11.003 - DOI - PubMed
-
- Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504. 10.1056/NEJMoa040583 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
