Montelukast in paediatric asthma and allergic rhinitis: a systematic review and meta-analysis
- PMID: 37852659
- PMCID: PMC10582929
- DOI: 10.1183/16000617.0124-2023
Montelukast in paediatric asthma and allergic rhinitis: a systematic review and meta-analysis
Abstract
Background: We aim to assess the impact of montelukast on paediatric patients with asthma/allergic rhinitis, measured using patient-reported outcome measures, compared with other treatments or placebo.
Methods: Protocol registration CRD42020216098 (www.crd.york.ac.uk/PROSPERO). MEDLINE and Embase databases were used to conduct the search. Two authors independently selected studies and extracted data, and a third reviewer resolved discrepancies. Meta-analyses were constructed to estimate the standardised mean difference (SMD) using a random-effects model.
Results: Out of 3937 articles identified, 49 studies met the inclusion criteria, mostly randomised clinical trials (sample sizes: 21-689 patients). The SMD of change pooled estimators for the global, mental and physical domains of health-related quality of life were not statistically significant. For daytime and night-time symptoms scores, the SMD (95% CI) was in favour of inhaled corticosteroids (-0.12, -0.20- -0.05 and -0.23, -0.41- -0.06, respectively). The pooled estimator for global asthma symptoms was better for montelukast when compared with placebo (0.90, 0.44-1.36).
Conclusions: The synthesis of the available evidence suggests that, in children and adolescents, montelukast was effective in controlling asthma symptoms when compared with placebo, but inhaled corticosteroids were superior in controlling symptoms, especially at night-time. These findings of our systematic review concur with current guidelines for asthma treatment.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: All authors have nothing to disclose.
Figures
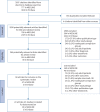




References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. doi: 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
-
- Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. Available from: http://ginasthma.org/
-
- U.S. Department of Health and Human Services . 2020 Updates to the Asthma Management Guidelines. 2020. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focu... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
