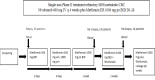
Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer
- PMID: 37852737
- PMCID: PMC10603338
- DOI: 10.1136/jitc-2023-007235
Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer
Abstract
Background: Preclinical studies showed metformin reduces exhaustion of tumor-infiltrating lymphocytes and potentiates programmed cell death protein-1 (PD-1) blockade. We hypothesized that metformin with nivolumab would elicit potent antitumor and immune modulatory activity in metastatic microsatellite stable (MSS) colorectal cancer (CRC). We evaluated this hypothesis in a phase II study.
Methods: Nivolumab (480 mg) was administered intravenously every 4 weeks while metformin (1000 mg) was given orally, two times per day following a 14-day metformin only lead-in phase. Patients ≥18 years of age, with previously treated, stage IV MSS CRC, and Eastern Cooperative Oncology Group 0-1, having received no prior anti-PD-1 agent were eligible. The primary endpoint was overall response rate with secondary endpoints of overall survival (OS) and progression-free survival (PFS). Correlative studies using paired pretreatment/on-treatment biopsies and peripheral blood evaluated a series of immune biomarkers in the tumor microenvironment and systemic circulation using ChipCytometry and flow cytometry.
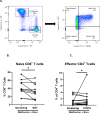
Results: A total of 24 patients were enrolled, 6 patients were replaced per protocol, 18 patients had evaluable disease. Of the 18 evaluable patients, 11/18 (61%) were women and the median age was 58 (IQR 50-67). Two patients had stable disease, but no patients had objective response, hence the study was stopped for futility. Median OS and PFS was 5.2 months (95% CI (3.2 to 11.7)) and 2.3 months (95% CI (1.7 to 2.3)). Most common grade 3/4 toxicities: Anemia (n=2), diarrhea (n=2), and fever (n=2). Metformin alone failed to increase the infiltration of T-cell subsets in the tumor, but combined metformin and nivolumab increased percentages of tumor-infiltrating leukocytes (p=0.031). Dual treatment also increased Tim3+ levels in patient tissues and decreased naïve CD8+T cells (p=0.0475).
Conclusions: Nivolumab and metformin were well tolerated in patients with MSS CRC but had no evidence of efficacy. Correlative studies did not reveal an appreciable degree of immune modulation from metformin alone, but showed trends in tumorous T-cell infiltration as a result of dual metformin and PD-1 blockade despite progression in a majority of patients.
Trial registration: ClinicalTrials.gov NCT03800602.
Keywords: Clinical Trials, Phase II as Topic; Drug Therapy, Combination; Immunotherapy; Translational Medical Research.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MA has consulted for Eisai, Ipsen, Exelixis, GSK, QED, Isofol, Curio Science, AstraZeneca and Genentech and has received research funding from Tesaro (Inst), RedHill Biopharma Limited (Inst), Polaris (Inst), Bristol-Myers Squibb-Ono Pharmaceutical (Inst), Xencor (Inst), Merck Sharp & Dohme (Inst), Eisai (Inst), GSK (Inst). BER has consulted for AZ, Ipsen, Exelixis, Seagen and received research funding from BMS, Merck, AZ, Novartis, EUSA, adptamune, Bayer, Exelixis. GBL has consulted for ProDa Biotech, LLC and received compensation. GBL has received research funding through a sponsored research agreement between Emory University and Merck and Co., Bristol-Myers Squibb, Boerhinger-Ingelheim, and Vaccinex. CW has consulted for Array Biopharma, Natera, Seagen, Pfizer, Daiichi Sankyo/Lilly and has received research funding (trial funding to institution) through Pfizer, Hutchmed and Seagen. Employment/leadership/stock and other ownership—nothing to disclose. Honoraria: Cancer Treatment Centers of America, Medscape. WLS is on the advisory board of Mylan, BMS, Esai, Tesaro, Blueprints, Seagen and a member of the speaker’s bureau of Guardant health, Cumberland therapeutics, Seagen. His research has been funded by GSK, Eli Lilly, Tesaro. BO has received research funding through a sponsored research agreement between Emory University and Boehringer Ingelheim. OBA has consulted for Ipsen Pharmaceuticals, Aadi Bioscience, Taiho, Pfizer, Seagen Inc. Bristol-Myers Squibb and AstraZeneca. He has received research funding from Taiho Oncology, Ipsen Pharmaceuticals, GSK, Bristol-Myers Squibb, PCI Biotech AS, ASCO, Calithera Biosciences, Inc., SynCore Biotechnology Co. Ltd., Suzhou Transcenta Therapeutics Co., Ltd, Corcept Therapeutics Inc., Hutchison MediPharma, Boehringer Ingelheim, Xencor Inc., Cue Biopharma, Inc., Merck, Syros Pharmaceuticals Inc., Inhibitex Inc, Arcus Biosciences Inc. and ImmunoGen. MD has consulted for Novartis and Guardant Health. The rest of the authors have no conflicts of interest to disclose.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
