FSH and ApoE4 contribute to Alzheimer's disease-like pathogenesis via C/EBPβ/δ-secretase in female mice
- PMID: 37852961
- PMCID: PMC10584868
- DOI: 10.1038/s41467-023-42282-7
FSH and ApoE4 contribute to Alzheimer's disease-like pathogenesis via C/EBPβ/δ-secretase in female mice
Abstract
Alzheimer's disease (AD) is the most common dementia. It is known that women with one ApoE4 allele display greater risk and earlier onset of AD compared with men. In mice, we previously showed that follicle-stimulating hormone (FSH), a gonadotropin that rises in post-menopausal females, activates its receptor FSHR in the hippocampus, to drive AD-like pathology and cognitive impairment. Here we show in mice that ApoE4 and FSH jointly trigger AD-like pathogenesis by activating C/EBPβ/δ-secretase signaling. ApoE4 and FSH additively activate C/EBPβ/δ-secretase pathway that mediates APP and Tau proteolytic fragmentation, stimulating Aβ and neurofibrillary tangles. Ovariectomy-provoked AD-like pathologies and cognitive defects in female ApoE4-TR mice are ameliorated by anti-FSH antibody treatment. FSH administration facilitates AD-like pathologies in both young male and female ApoE4-TR mice. Furthermore, FSH stimulates AD-like pathologies and cognitive defects in ApoE4-TR mice, but not ApoE3-TR mice. Our findings suggest that in mice, augmented FSH in females with ApoE4 but not ApoE3 genotype increases vulnerability to AD-like process by activating C/EBPβ/δ-secretase signalling.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
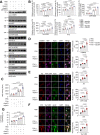
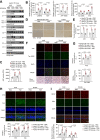

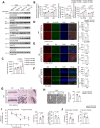
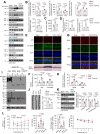


References
-
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997;18:739–773. - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997;15:201–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

