Bacteriophages suppress CRISPR-Cas immunity using RNA-based anti-CRISPRs
- PMID: 37853129
- PMCID: PMC10651486
- DOI: 10.1038/s41586-023-06612-5
Bacteriophages suppress CRISPR-Cas immunity using RNA-based anti-CRISPRs
Abstract
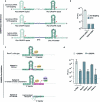
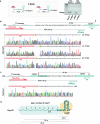
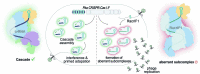
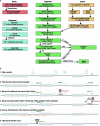
Many bacteria use CRISPR-Cas systems to combat mobile genetic elements, such as bacteriophages and plasmids1. In turn, these invasive elements have evolved anti-CRISPR proteins to block host immunity2,3. Here we unveil a distinct type of CRISPR-Cas Inhibition strategy that is based on small non-coding RNA anti-CRISPRs (Racrs). Racrs mimic the repeats found in CRISPR arrays and are encoded in viral genomes as solitary repeat units4. We show that a prophage-encoded Racr strongly inhibits the type I-F CRISPR-Cas system by interacting specifically with Cas6f and Cas7f, resulting in the formation of an aberrant Cas subcomplex. We identified Racr candidates for almost all CRISPR-Cas types encoded by a diverse range of viruses and plasmids, often in the genetic context of other anti-CRISPR genes5. Functional testing of nine candidates spanning the two CRISPR-Cas classes confirmed their strong immune inhibitory function. Our results demonstrate that molecular mimicry of CRISPR repeats is a widespread anti-CRISPR strategy, which opens the door to potential biotechnological applications6.
© 2023. The Author(s).
Conflict of interest statement
R.P.-R., S.C.-W., J.R. and S.J.S. are inventors on a patent related to methods for modulating Cas-effector activity. D.M.-M., P.C.F. and R.D.F. are inventors on patents relating to CRISPR–Cas technologies and uses thereof. J.S.M. declares no competing interests.
Figures








Comment in
-
Viruses use RNA decoys to thwart CRISPR defences.Nature. 2023 Nov;623(7987):490-491. doi: 10.1038/d41586-023-03133-z. Nature. 2023. PMID: 37853195 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

