Elucidating regulatory processes of intense physical activity by multi-omics analysis
- PMID: 37853489
- PMCID: PMC10583322
- DOI: 10.1186/s40779-023-00477-5
Elucidating regulatory processes of intense physical activity by multi-omics analysis
Abstract
Background: Physiological and biochemical processes across tissues of the body are regulated in response to the high demands of intense physical activity in several occupations, such as firefighting, law enforcement, military, and sports. A better understanding of such processes can ultimately help improve human performance and prevent illnesses in the work environment.
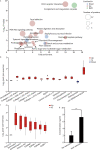
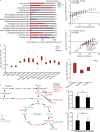
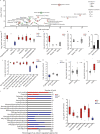
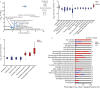
Methods: To study regulatory processes in intense physical activity simulating real-life conditions, we performed a multi-omics analysis of three biofluids (blood plasma, urine, and saliva) collected from 11 wildland firefighters before and after a 45 min, intense exercise regimen. Omics profiles post- versus pre-exercise were compared by Student's t-test followed by pathway analysis and comparison between the different omics modalities.
Results: Our multi-omics analysis identified and quantified 3835 proteins, 730 lipids and 182 metabolites combining the 3 different types of samples. The blood plasma analysis revealed signatures of tissue damage and acute repair response accompanied by enhanced carbon metabolism to meet energy demands. The urine analysis showed a strong, concomitant regulation of 6 out of 8 identified proteins from the renin-angiotensin system supporting increased excretion of catabolites, reabsorption of nutrients and maintenance of fluid balance. In saliva, we observed a decrease in 3 pro-inflammatory cytokines and an increase in 8 antimicrobial peptides. A systematic literature review identified 6 papers that support an altered susceptibility to respiratory infection.
Conclusion: This study shows simultaneous regulatory signatures in biofluids indicative of homeostatic maintenance during intense physical activity with possible effects on increased infection susceptibility, suggesting that caution against respiratory diseases could benefit workers on highly physical demanding jobs.
Keywords: Biofluids; Human performance; Immunity; Intense exercise; Metabolism; Multi-omics analysis.
© 2023. People´s Military Medical Press.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Friganovic A, Selic P, Ilic B, Sedic B. Stress and burnout syndrome and their associations with coping and job satisfaction in critical care nurses: a literature review. Psychiatr Danub. 2019;31(Suppl 1):21–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

