The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial
- PMID: 37853490
- PMCID: PMC10585921
- DOI: 10.1186/s13054-023-04663-8
The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial
Abstract
Background: Based on low-quality evidence, current nutrition guidelines recommend the delivery of high-dose protein in critically ill patients. The EFFORT Protein trial showed that higher protein dose is not associated with improved outcomes, whereas the effects in critically ill patients who developed acute kidney injury (AKI) need further evaluation. The overall aim is to evaluate the effects of high-dose protein in critically ill patients who developed different stages of AKI.
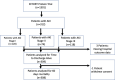
Methods: In this post hoc analysis of the EFFORT Protein trial, we investigated the effect of high versus usual protein dose (≥ 2.2 vs. ≤ 1.2 g/kg body weight/day) on time-to-discharge alive from the hospital (TTDA) and 60-day mortality and in different subgroups in critically ill patients with AKI as defined by the Kidney Disease Improving Global Outcomes (KDIGO) criteria within 7 days of ICU admission. The associations of protein dose with incidence and duration of kidney replacement therapy (KRT) were also investigated.
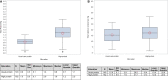
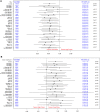
Results: Of the 1329 randomized patients, 312 developed AKI and were included in this analysis (163 in the high and 149 in the usual protein dose group). High protein was associated with a slower time-to-discharge alive from the hospital (TTDA) (hazard ratio 0.5, 95% CI 0.4-0.8) and higher 60-day mortality (relative risk 1.4 (95% CI 1.1-1.8). Effect modification was not statistically significant for any subgroup, and no subgroups suggested a beneficial effect of higher protein, although the harmful effect of higher protein target appeared to disappear in patients who received kidney replacement therapy (KRT). Protein dose was not significantly associated with the incidence of AKI and KRT or duration of KRT.
Conclusions: In critically ill patients with AKI, high protein may be associated with worse outcomes in all AKI stages. Recommendation of higher protein dosing in AKI patients should be carefully re-evaluated to avoid potential harmful effects especially in patients who were not treated with KRT.
Trial registration: This study is registered at ClinicalTrials.gov (NCT03160547) on May 17th 2017.
Keywords: Acute kidney injury; Critical illness; Nutrition support; Protein; Randomized trial; Registry trial.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors do not report a competing interests inside, or related to the present work.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

