Tenapanor Improves Long-Term Control of Hyperphosphatemia in Patients Receiving Maintenance Dialysis: the NORMALIZE Study
- PMID: 37853560
- PMCID: PMC10695649
- DOI: 10.34067/KID.0000000000000280
Tenapanor Improves Long-Term Control of Hyperphosphatemia in Patients Receiving Maintenance Dialysis: the NORMALIZE Study
Abstract
Key Points:
Tenapanor is a first-in-class, minimally systemic sodium–hydrogen exchanger 3 inhibitor with a mechanism of action distinct from phosphate binders.
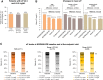
Tenapanor alone or with phosphate binders led to 35%–49% of patients achieving serum phosphate ≤4.5 mg/dl over an 18-month period versus 22% at baseline.
Tenapanor alone or with phosphate binders may help adults with CKD on maintenance dialysis achieve normal serum phosphate concentrations.
Background: Most patients with ESKD and hyperphosphatemia have difficulty controlling serum phosphate (sP) concentrations despite maintenance dialysis, dietary restriction, and phosphate binder treatment. NORMALIZE evaluated the efficacy and safety of tenapanor 30 mg twice daily alone or in combination with phosphate binders to achieve sP within the adult population reference range (2.5–4.5 mg/dl).
Methods: Patients who completed the Phase 3 PHREEDOM study could enroll in NORMALIZE. Patients enrolled in NORMALIZE who had received tenapanor during the PHREEDOM study (n=111) added sevelamer carbonate if sP was >4.5 mg/dl. Patients who had received sevelamer carbonate during the PHREEDOM study (n=61) added tenapanor and decreased sevelamer carbonate if sP was ≤4.5 mg/dl, per protocol titration schedule. Patients were followed in NORMALIZE for up to 18 months. We assessed efficacy in the full analysis set, defined as patients who received ≥1 dose of study drug and had ≥1 post-treatment sP measurement (n=171). We assessed safety in all patients who received ≥1 dose of study drug (n=172).
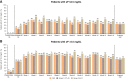
Results: At the end point visit, 57 of 171 patients (33%) in the full analysis set achieved sP between 2.5 and 4.5 mg/dl. Eight of 23 patients (35%) who were on tenapanor alone at the end point visit achieved sP between 2.5 and 4.5 mg/dl. The mean reduction from PHREEDOM baseline to end of NORMALIZE in sP was 2.0 mg/dl. Serum intact fibroblast growth factor-23 was significantly reduced; serum intact parathyroid hormone was significantly reduced among patients with intact parathyroid hormone ≥300 pg/ml at PHREEDOM baseline. The most commonly reported treatment-emergent adverse event was diarrhea in 38 of 172 patients (22%), which led to tenapanor discontinuation in four patients (2%).
Conclusions: Tenapanor alone or in combination with phosphate binders helped adult patients on maintenance dialysis achieve normal sP concentrations. Safety was consistent with previous studies of tenapanor.
Clinical trial registry name and registration number:
A Long-Term Study to Evaluate the Ability of Tenapanor Alone or in Combination With Sevelamer to Treat to Goal Serum Phosphorus in Patients With ESKD on Dialysis (NORMALIZE),
Trial registration: ClinicalTrials.gov NCT03427125 NCT03988920 NCT02675998 NCT03824597 NCT04549597.
Conflict of interest statement
G.M. Chertow reports the following—consultancy: Akebia, Ardelyx, Inc., AstraZeneca, Calico, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; ownership interest: Ardelyx, Inc., CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, PuraCath, Renibus, and Unicycive; Research Funding: CSL Behring, NIAID, and NIDDK; advisory or leadership Role: Board of Directors, Satellite Healthcare, Co-Editor,
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

