Similarly slow diffusion of BAM and SecYEG complexes in live E. coli cells observed with 3D spt-PALM
- PMID: 37853695
- PMCID: PMC10698321
- DOI: 10.1016/j.bpj.2023.10.017
Similarly slow diffusion of BAM and SecYEG complexes in live E. coli cells observed with 3D spt-PALM
Abstract
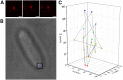
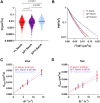
The β-barrel assembly machinery (BAM) complex is responsible for inserting outer membrane proteins (OMPs) into the Escherichia coli outer membrane. The SecYEG translocon inserts inner membrane proteins into the inner membrane and translocates both soluble proteins and nascent OMPs into the periplasm. Recent reports describe Sec possibly playing a direct role in OMP biogenesis through interactions with the soluble polypeptide transport-associated (POTRA) domains of BamA (the central OMP component of BAM). Here we probe the diffusion behavior of these protein complexes using photoactivatable super-resolution localization microscopy and single-particle tracking in live E. coli cells of BAM and SecYEG components BamA and SecE and compare them to other outer and inner membrane proteins. To accurately measure trajectories on the highly curved cell surface, three-dimensional tracking was performed using double-helix point-spread function microscopy. All proteins tested exhibit two diffusive modes characterized by "slow" and "fast" diffusion coefficients. We implement a diffusion coefficient analysis as a function of the measurement lag time to separate positional uncertainty from true mobility. The resulting true diffusion coefficients of the slow and fast modes showed a complete immobility of full-length BamA constructs in the time frame of the experiment, whereas the OMP OmpLA displayed a slow diffusion consistent with the high viscosity of the outer membrane. The periplasmic POTRA domains of BamA were found to anchor BAM to other cellular structures and render it immobile. However, deletion of individual distal POTRA domains resulted in increased mobility, suggesting that these domains are required for the full set of cellular interactions. SecE diffusion was much slower than that of the inner membrane protein PgpB and was more like OMPs and BamA. Strikingly, SecE diffused faster upon POTRA domain deletion. These results are consistent with the existence of a BAM-SecYEG trans-periplasmic assembly in live E. coli cells.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

