Impact of COVID-19 and vaccination campaign on 1,755 systemic sclerosis patients during first three years of pandemic. Possible risks for individuals with impaired immunoreactivity to vaccine, ongoing immunomodulating treatments, and disease-related lung involvement during the next pandemic phase
- PMID: 37854035
- PMCID: PMC10580042
- DOI: 10.1016/j.jtauto.2023.100212
Impact of COVID-19 and vaccination campaign on 1,755 systemic sclerosis patients during first three years of pandemic. Possible risks for individuals with impaired immunoreactivity to vaccine, ongoing immunomodulating treatments, and disease-related lung involvement during the next pandemic phase
Abstract
Introduction: The impact of COVID-19 pandemic represents a serious challenge for 'frail' patients' populations with inflammatory autoimmune systemic diseases such as systemic sclerosis (SSc). We investigated the prevalence and severity of COVID-19, as well the effects of COVID-19 vaccination campaign in a large series of SSc patients followed for the entire period (first 38 months) of pandemic.
Patients and method: This prospective survey study included 1755 unselected SSc patients (186 M, 1,569F; mean age 58.7 ± 13.4SD years, mean disease duration 8.8 ± 7.3SD years) recruited in part by telephone survey at 37 referral centers from February 2020 to April 2023. The following parameters were carefully evaluated: i. demographic, clinical, serological, and therapeutical features; ii. prevalence and severity of COVID-19; and iii. safety, immunogenicity, and efficacy of COVID-19 vaccines.
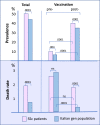
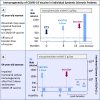
Results: The prevalence of COVID-19 recorded during the whole pandemic was significantly higher compared to Italian general population (47.3 % vs 43.3 %, p < 0.000), as well the COVID-19-related mortality (1.91 % vs 0.72 %, p < 0.001). As regards the putative prognostic factors of worse outcome, COVID-19 positive patients with SSc-related interstitial lung involvement showed significantly higher percentage of COVID-19-related hospitalization compared to those without (5.85 % vs 1.73 %; p < 0.0001), as well as of mortality rate (2.01 % vs 0.4 %; p = 0.002). Over half of patients (56.3 %) received the first two plus one booster dose of vaccine; while a fourth dose was administered to 35.6 %, and only few of them (1.99 %) had five or more doses of vaccine. Of note, an impaired seroconversion was recorded in 25.6 % of individuals after the first 2 doses of vaccine, and in 8.4 % of patients also after the booster dose. Furthermore, the absence of T-cell immunoreactivity was observed in 3/7 patients tested by QuantiFERON® SARSCoV-2 Starter Set (Qiagen). The efficacy of vaccines, evaluated by comparing the COVID-19-related death rate recorded during pre- and post-vaccination pandemic periods, revealed a quite stable outcome in SSc patients (death rate from 2.54 % to 1.76 %; p = ns), despite the significant drop of mortality observed in the Italian general population (from 2.95 % to 0.29 %; p < 0.0001).
Conclusions: An increased COVID-19 prevalence and mortality rate was recorded in SSc patients; moreover, the efficacy of vaccines in term of improved outcomes was less evident in SSc compared to Italian general population. This discrepancy might be explained by concomitant adverse prognostic factors: increased rate of non-responders to vaccine in SSc series, low percentage of individuals with four or more doses of vaccine, ongoing immunomodulating treatments, disease-related interstitial lung disease, and/or reduced preventive measures in the second half of pandemic. A careful monitoring of response to COVID-19 vaccines together with adequate preventive/therapeutical strategies are highly recommendable in the near course of pandemic in this frail patients' population.
Keywords: COVID-19; COVID-19 vaccine; Interstitial lung disease; SARS-CoV-2; Scleroderma; Systemic sclerosis.
© 2023 Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Alessandro Antonelli reports financial support was provided by Italian 10.13039/100009647Ministry of Health, Ricerca Finalizzata (RF-2021-12374986) Destinatario istituzionale: Regione Toscana. Unità Operative:U.O.1: Azienda Ospedaliero-Universitaria Pisana; U.O.2: Azienda Ospedaliero-Universitaria Aldo Moro Bari; U.O.3: Azienda Ospedaliero-Universitaria Modena, CUP Master: D55E22000670001.
Figures


References
-
- Costa L., Tasso M., Scotti N., Mostacciuolo E., Girolimetto N., Foglia F., et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort. Ann. Rheum. Dis. 2020;80:e46. - PubMed
-
- Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. - PubMed
-
- Bozzalla Cassione E., Zanframundo G., Biglia A., Codullo V., Montecucco C., Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann. Rheum. Dis. 2020;79:1382–1383. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

