Besnoitia besnoiti-induced neutrophil clustering and neutrophil extracellular trap formation depend on P2X1 purinergic receptor signaling
- PMID: 37854595
- PMCID: PMC10579820
- DOI: 10.3389/fimmu.2023.1244068
Besnoitia besnoiti-induced neutrophil clustering and neutrophil extracellular trap formation depend on P2X1 purinergic receptor signaling
Abstract
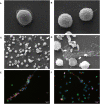
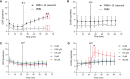
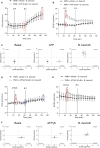
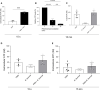
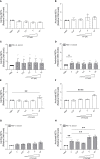
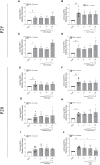
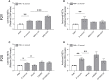
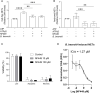
Bovine besnoitiosis is a re-emerging cattle disease caused by the cyst-forming apicomplexan parasite Besnoitia besnoiti. Neutrophil extracellular trap (NET) formation represents an efficient innate immune mechanism of polymorphonuclear neutrophils (PMN) against apicomplexan parasites, including B. besnoiti. PMN purinergic signaling was proposed as a critical factor for NET formation. One important purinergic ligand is ATP, which is recognized as a danger signal and released into the extracellular space acting as an autocrine/paracrine signaling molecule. ATP-driven effects on PMN via the nucleotide P2 receptor family include chemotaxis, reactive oxygen species (ROS) production, and NET formation. So far, data on both PMN ATP concentrations and the role of ATP as a key modulator of purinergic signaling in B. besnoiti tachyzoite-triggered bovine NETosis is scarce. Current data showed that B. besnoiti tachyzoite exposure to bovine PMN neither changed total PMN ATP nor extracellular ATP quantities even though it significantly triggered NET formation. Moreover, B. besnoiti tachyzoite-exposed PMN revealed enhanced oxygen consumption rates (OCR) as quantified by the Seahorse metabolic analyzer. Exogenous supplementation of ATP or non-hydrolizable ATP (ATPγS) led to increased extracellular acidification rates (ECAR) but failed to alter tachyzoite-induced oxidative responses (OCR) in exposed PMN. In addition, exogenous supplementation of ATPγS, but not of ATP, boosted B. besnoiti tachyzoite-induced anchored NET formation. Referring to purinergic signaling, B. besnoiti tachyzoite-triggered anchored NET formation revealed P2X1 purinergic as receptor-dependent since it was blocked by the P2X1 inhibitor NF449 at an IC50 of 1.27 µM. In contrast, antagonists of P2Y2, P2Y6, P2X4, and P2X7 purinergic receptors all failed to affect parasite-driven NETosis. As an interesting finding, we additionally observed that B. besnoiti tachyzoite exposure induced PMN clustering in a P2X1-dependent manner. Thus, we identified P2X1 purinergic receptor as a pivotal molecule for both B. besnoiti tachyzoite-induced PMN clustering and anchored NET formation.
Keywords: ATP; Besnoitia besnoiti; NET formation; PMN; immunometabolism; purinergic receptors.
Copyright © 2023 Espinosa, Conejeros, Rojas-Barón, Hermosilla and Taubert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bovine PMN responses to extracellular vesicles released by Besnoitia besnoiti tachyzoites and B. besnoiti-infected host cells.Front Immunol. 2024 Dec 19;15:1509355. doi: 10.3389/fimmu.2024.1509355. eCollection 2024. Front Immunol. 2024. PMID: 39749330 Free PMC article.
-
The CAMKK/AMPK Pathway Contributes to Besnoitia besnoiti-Induced NETosis in Bovine Polymorphonuclear Neutrophils.Int J Mol Sci. 2024 Aug 2;25(15):8442. doi: 10.3390/ijms25158442. Int J Mol Sci. 2024. PMID: 39126009 Free PMC article.
-
Metabolic requirements of Besnoitia besnoiti tachyzoite-triggered NETosis.Parasitol Res. 2020 Feb;119(2):545-557. doi: 10.1007/s00436-019-06543-z. Epub 2019 Nov 28. Parasitol Res. 2020. PMID: 31782011
-
A century of bovine besnoitiosis: an unknown disease re-emerging in Europe.Trends Parasitol. 2013 Aug;29(8):407-15. doi: 10.1016/j.pt.2013.06.002. Epub 2013 Jul 3. Trends Parasitol. 2013. PMID: 23830145 Review.
-
Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae).Infect Genet Evol. 2011 Oct;11(7):1564-76. doi: 10.1016/j.meegid.2011.08.006. Epub 2011 Aug 30. Infect Genet Evol. 2011. PMID: 21906696 Review.
Cited by
-
Bovine PMN responses to extracellular vesicles released by Besnoitia besnoiti tachyzoites and B. besnoiti-infected host cells.Front Immunol. 2024 Dec 19;15:1509355. doi: 10.3389/fimmu.2024.1509355. eCollection 2024. Front Immunol. 2024. PMID: 39749330 Free PMC article.
-
Cellular immune responses of bovine polymorphonuclear neutrophils to Calicophoron daubneyi.Front Immunol. 2025 Feb 13;16:1515419. doi: 10.3389/fimmu.2025.1515419. eCollection 2025. Front Immunol. 2025. PMID: 40018045 Free PMC article.
-
No Evidence of Neutrophil Response Modulation in Goats after Immunization against Paratuberculosis with a Heat-Inactivated Vaccine.Animals (Basel). 2024 Jun 5;14(11):1694. doi: 10.3390/ani14111694. Animals (Basel). 2024. PMID: 38891741 Free PMC article.
-
AMPK and CAMKK activation participate in early events of Toxoplasma gondii-triggered NET formation in bovine polymorphonuclear neutrophils.Front Vet Sci. 2025 Mar 18;12:1557509. doi: 10.3389/fvets.2025.1557509. eCollection 2025. Front Vet Sci. 2025. PMID: 40171409 Free PMC article.
-
The CAMKK/AMPK Pathway Contributes to Besnoitia besnoiti-Induced NETosis in Bovine Polymorphonuclear Neutrophils.Int J Mol Sci. 2024 Aug 2;25(15):8442. doi: 10.3390/ijms25158442. Int J Mol Sci. 2024. PMID: 39126009 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

