Identification of an APOE ε4-specific blood-based molecular pathway for Alzheimer's disease risk
- PMID: 37854772
- PMCID: PMC10579631
- DOI: 10.1002/dad2.12490
Identification of an APOE ε4-specific blood-based molecular pathway for Alzheimer's disease risk
Abstract
Introduction: The precise apolipoprotein E (APOE) ε4-specific molecular pathway(s) for Alzheimer's disease (AD) risk are unclear.
Methods: Plasma protein modules/cascades were analyzed using weighted gene co-expression network analysis (WGCNA) in the Alzheimer's Disease Neuroimaging Initiative study. Multivariable regression analyses were used to examine the associations among protein modules, AD diagnoses, cerebrospinal fluid (CSF) phosphorylated tau (p-tau), and brain glucose metabolism, stratified by APOE genotype.
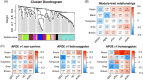
Results: The Green Module was associated with AD diagnosis in APOE ε4 homozygotes. Three proteins from this module, C-reactive protein (CRP), complement C3, and complement factor H (CFH), had dose-dependent associations with CSF p-tau and cognitive impairment only in APOE ε4 homozygotes. The link among these three proteins and glucose hypometabolism was observed in brain regions of the default mode network (DMN) in APOE ε4 homozygotes. A Framingham Heart Study validation study supported the findings for AD.
Discussion: The study identifies the APOE ε4-specific CRP-C3-CFH inflammation pathway for AD, suggesting potential drug targets for the disease.Highlights: Identification of an APOE ε4 specific molecular pathway involving blood CRP, C3, and CFH for the risk of AD.CRP, C3, and CFH had dose-dependent associations with CSF p-Tau and brain glucose hypometabolism as well as with cognitive impairment only in APOE ε4 homozygotes.Targeting CRP, C3, and CFH may be protective and therapeutic for AD onset in APOE ε4 carriers.
Keywords: Alzheimer's disease; C‐reactive protein; age‐related macular degeneration; amyloid beta peptide; apolipoprotein E; cerebrospinal fluid phosphorylated tau; cognitive impairment; complement C3; complement factor H; hypometabolic convergence index; positron emission tomography.
© 2023 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no competing interests. The sponsor institutes did not play any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Author disclosures are available in the supporting information.
Figures




References
-
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278(16):1349‐1356. - PubMed
-
- Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. American J Clin Nutrit. 2000;72(5):1170‐1178. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
