Avidin cooperative allosterism upon binding biotin observed by differential changes in intrinsic fluorescence
- PMID: 37854942
- PMCID: PMC10579862
- DOI: 10.1016/j.bbrep.2023.101554
Avidin cooperative allosterism upon binding biotin observed by differential changes in intrinsic fluorescence
Abstract
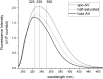
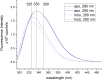
Similar to streptavidin, the binding of biotin by avidin does not appear to be cooperative in the traditional sense of altered binding strength, though it appears to be cooperative in terms of ligand induced structural communication across subunits in the protein as previously shown for streptavidin. In this work we provide data from intrinsic tryptophan fluorescence as evidence of a cooperative structural change. The technique involves examination of the changes in fluorescence emission corresponding to the various tryptophan populations accompanying avidin-biotin binding. We note that the 335 nm emission population (i.e. more hydrophobic local environment) saturates prior to full ligation and the saturation of the 350 nm emission population commonly used in standard binding activity assays. We also note that total integrated fluorescence emission and peak height during the titration of ligand into streptavidin also reach saturation prior to the 4:1 stoichiometric end point. Unique to avidin and distinct from the behavior of streptavidin described in our prior work, the wavelength of maximum emission and full width at half maximum (FWHM) data do not saturate prior to the 4:1 stoichiometric end point. Avidin also exhibited larger FWHM for both apo and holo forms suggesting greater heterogeneity in local tryptophan environments, as compared to streptavidin. Taken together, the data suggests that the binding of the first 3 biotins effect greater structural changes in the protein than the final ligand in a similar way for avidin and streptavidin.
Keywords: Allosterism; Avidin; Protein-ligand binding; Tryptophan fluorescence.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Kurzban G.P., Gitlin G., Bayer E.A., Wilchek M., Horowitz P.M. Biotin binding changes the conformation and decreases tryptophan accessibility of streptavidin. J. Protein Chem. 1990;9(6):673–682. - PubMed
-
- Kurzban G.P., Bayer E.A., Wilchek M., Horowitz P.M. The quaternary structure of streptavidin in urea. J. Biol. Chem. 1991;266(22):14470–14477. - PubMed
-
- Joseph R. Springer; 2017. Lakowicz. Principles Of Fluorescence Spectroscopy.
-
- González M., Bagatolli L.A., Echabe I., Arrondo J.L., Argaraña C.E., Cantor C.R., Fidelio G.D. Interaction of biotin with streptavidin. Thermostability and conformational changes upon binding. J. Biol. Chem. 1997;272(17):11288–11294. - PubMed
LinkOut - more resources
Full Text Sources

