Effect of the addition of a mental health specialist for evaluation of undiagnosed patients in centres for rare diseases (ZSE-DUO): a prospective, controlled trial with a two-phase cohort design
- PMID: 37855024
- PMCID: PMC10579280
- DOI: 10.1016/j.eclinm.2023.102260
Effect of the addition of a mental health specialist for evaluation of undiagnosed patients in centres for rare diseases (ZSE-DUO): a prospective, controlled trial with a two-phase cohort design
Abstract
Background: People with complex symptomatology but unclear diagnosis presenting to a centre for rare diseases (CRD) may present with mental (co-)morbidity. We hypothesised that combining an expert in somatic medicine with a mental health specialist working in tandem will improve the diagnostic outcome.
Methods: Patients aged 12 years and older who presented to one of the 11 participating German CRDs with an unknown diagnosis were recruited into this prospective cohort trial with a two-phase cohort design. From October 1, 2018 to September 30, 2019, participants were allocated to standard care (SC, N = 684), and from October 1, 2019 to January 31, 2021 to innovative care (IC, N = 695). The cohorts consisted mainly of adult participants with only a minority of children included (N = 67). IC included the involvement of a mental health specialist in all aspects of care (e.g., assessing medical records, clinic visits, telehealth care, and case conferences). Clinicaltrials.gov identifier: NCT03563677.
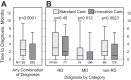
Findings: The proportion of patients with diagnoses established within 12 months after the first visit to the CRD explaining the entire symptomatology (primary outcome) was 19% (N = 131 of 672) in the SC and 42% (N = 286 of 686) in the IC cohort (OR adjusted for centre effects 3.45 [95% CrI: 1.99-5.65]). The difference was mainly due to a higher prevalence of mental disorders and non-rare somatic diseases in the IC cohort. The median time to explaining diagnoses was one month shorter with IC (95% CrI: 1-2), and significantly more patients could be referred to local regular care in the IC (27.5%; N = 181 of 659) compared to the SC (12.3%; N = 81 of 658) cohort (OR adjusted for centre effects 2.70 [95% CrI: 2.02-3.60]). At 12-month follow-up, patient satisfaction with care was significantly higher in the IC compared to the SC cohort, while quality of life was not different between cohorts.
Interpretation: Our findings suggested that including a mental health specialist in the entire evaluation process of CRDs for undiagnosed adolescents and adults should become an integral part of the assessment of individuals with a suspected rare disease.
Funding: The study was funded by the Global Innovation Fund from the Joint Federal Committee in Germany (Innovationsfonds des Gemeinsamen Bundesausschusses), grant number 01NVF17031.
Keywords: Diagnostic services; Medically unexplained symptoms; Mental health; Patient care team; Rare diseases.
© 2023 The Author(s).
Conflict of interest statement
HH, MB and TM report funding from the Bavarian State Ministry of Science and the Arts paid to their institution to support projects on rare diseases unrelated to ZSE-DUO. HH also reports unrelated funding to his institution by the Federal Ministry of Education and Research, and honoraria from Springer Verlag, Takeda Pharma GmbH and Chiesi GmbH. JD reports funding to his institution from the German Research Association, German Secretary of Education and Research, and the Innovation Fund for several unrelated projects. GH's institution has received funding from the Innovation Fund for another project. OT's institution has received funding from the European Regional Development Fund, EU Horizon 2020, Leibniz Association, and the Federal Ministry of Education and Research. OT has received royalties as an author. CZ reports funding to her institution by the Federal Ministry of Education and Research and the European Union, and voluntary work for the advisory board of the severe chronic neutropenia international registry. All other authors declare no competing interests.
Figures



References
-
- Davies S.C. Annual report of the chief medical officer 2016, generation genome london. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploa... Available at:
-
- United Nations General Assembly Addressing the challenges of persons living with a rare disease and their families. 2021. https://www.rarediseasesinternational.org/wp-content/uploads/2022/01/Fin... Available at:
Associated data
LinkOut - more resources
Full Text Sources
Medical

