Safety and efficacy of inhaled interferon-β1a (SNG001) in adults with mild-to-moderate COVID-19: a randomized, controlled, phase II trial
- PMID: 37855026
- PMCID: PMC10579289
- DOI: 10.1016/j.eclinm.2023.102250
Safety and efficacy of inhaled interferon-β1a (SNG001) in adults with mild-to-moderate COVID-19: a randomized, controlled, phase II trial
Abstract
Background: With the emergence of SARS-CoV-2 variants resistant to monoclonal antibody therapies and limited global access to therapeutics, the evaluation of novel therapeutics to prevent progression to severe COVID-19 remains a critical need.
Methods: Safety, clinical and antiviral efficacy of inhaled interferon-β1a (SNG001) were evaluated in a phase II randomized controlled trial on the ACTIV-2/A5401 platform (ClinicalTrials.govNCT04518410). Adult outpatients with confirmed SARS-CoV-2 infection within 10 days of symptom onset were randomized and initiated either orally inhaled nebulized SNG001 given once daily for 14 days (n = 110) or blinded pooled placebo (n = 110) between February 10 and August 18, 2021.
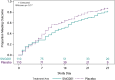
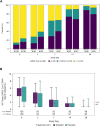
Findings: The proportion of participants reporting premature treatment discontinuation was 9% among SNG001 and 13% among placebo participants. There were no differences between participants who received SNG001 or placebo in the primary outcomes of treatment emergent Grade 3 or higher adverse events (3.6% and 8.2%, respectively), time to symptom improvement (median 13 and 9 days, respectively), or proportion with unquantifiable nasopharyngeal SARS-CoV-2 RNA at days 3 (28% [26/93] vs. 39% [37/94], respectively), 7 (65% [60/93] vs. 66% [62/94]) and 14 (91% [86/95] vs. 91% [83/81]). There were fewer hospitalizations with SNG001 (n = 1; 1%) compared with placebo (n = 7; 6%), representing an 86% relative risk reduction (p = 0.07). There were no deaths in either arm.
Interpretation: In this trial, SNG001 was safe and associated with a non-statistically significant decrease in hospitalization for COVID-19 pneumonia.
Funding: The ACTIV-2 platform study is funded by the NIH. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Keywords: ACTIV-2; COVID-19; Inhaled interferon; Randomized clinical trial; SARS-CoV-2.
© 2023 The Authors.
Conflict of interest statement
P.J. has received research funding to the institution from NIH/NIAID. K.W.C. has received research funding to the institution from NIH/NIAID and Merck Sharp & Dohme; has served as a consultant for Pardes Biosciences; and has received honoraria for CME from International Antiviral Society-USA. M.J.G. has received research funding to the institution from NIH/NIAID. M.D.H. has received research funding to the institution from NIH/NIAID. C.M. has received research funding to the institution from NIH/NIAID. M.J.M. has received research funding to the institution from NIH/NIAID. P.D.M. is an employee of Synairgen; Synairgen covered cost of supplying SNG001 and placebo for the study; Patents filed in relation to use of SNG001 to treat viral lung disease; Shareholder. A.C.J. report no competing interests. J.Z.L. has received research funding to the institution from NIH/NIAID. C.F. has received research funding to the institution from NIH/NIAID. C.Mc. has received research funding to the institution from NIH/NIAID. D.A.W. has received funding to the institution to support research and honoraria for advisory boards and consulting from Gilead Sciences, Eli Lilly, and Merck. E.S.D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV and research support through the institution from Gilead Sciences and GSK/ViiV. J.J.E. has received research funding to the institution from NIH/NIAID; is an ad hoc consultant to GSK/VIR, Merck, Gilead; data monitoring committee (DMC) chair for Adagio Phase III studies. W.F. has received research funding to the institution from Ridgeback Biopharmaceuticals, served on adjudication committees for Janssen, Syneos, served as a consultant for Roche and Merck, and has received honoraria for CME from Medlearning group. J.S.C. has received research funding to the institution from NIH/NIAID; has consulted for Merck and Company. U.S. has received research funding to the institution from NIH/NIAID and Pfizer; Scientific advisory board Burroughs Wellcome Fund. D.M.S. has received research funding to the institution from NIH/NIAID; has consulted for the following companies VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Lucira, Pharma Holdings, and Evidera.
Figures

References
-
- Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

