Impact of mild thrombocytopenia on bleeding and recurrent thrombosis in cancer
- PMID: 37855029
- PMCID: PMC11141682
- DOI: 10.3324/haematol.2023.284192
Impact of mild thrombocytopenia on bleeding and recurrent thrombosis in cancer
Abstract
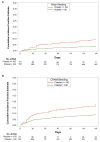
Thrombocytopenia occurs frequently in patients with cancer-associated thrombosis (CAT), however prospective evaluation of clinical outcomes following randomization to anticoagulants is limited. The HOKUSAI VTE Cancer study was a randomized, open-label, non-inferiority, phase III trial comparing dalteparin with edoxaban in CAT patients. This post hoc analysis of Hokusai VTE Cancer Study was performed to compare outcomes in patients with platelet count ≤100x109/L at one or more specified time points (baseline, 1-month, or 3-month) versus those without thrombocytopenia. Cumulative incidences at 180 days were calculated with death as a competing risk. The primary outcome was major bleeding; secondary outcomes were clinically relevant non-major bleeding (CRNMB), recurrent thrombosis, and survival. The analysis included 1,045 patients with primarily solid tumor malignancies (89%), median age 65 years, and 52% male. The thrombocytopenia group comprised 9.6% (N=101) of the cohort and relative to the non-thrombocytopenia cohort (N=944), experienced significantly higher major bleeding (9.0% vs. 4.0%, sub-distribution hazard ratio [SHR] =2.4; P=0.02) and CRNMB (17.9% vs. 9.6%, SHR=2.0; P=0.01). Thrombocytopenia did not impact recurrent venous thromboembolic event (VTE) (9.8% vs. 7.4%, SHR=1.3; P=0.37) nor overall mortality (21.8% vs. 26.0%, HR=0.9; P=0.48). Major bleeding was higher in patients with thrombocytopenia and gastrointestinal malignancies receiving edoxaban versus dalteparin (16.8% vs. 0; P<0.01) but similar for patients with other malignancies (P=0.30). In patients with hematologic malignances and thrombocytopenia major bleeding was higher for patients receiving dalteparin compared to edoxaban (19.0% vs. 0; P<0.01). Mild thrombocytopenia was associated with a doubling in risk of major hemorrhage in patients receiving anticoagulation for CAT. Bleeding risk for edoxaban and dalteparin varied in gastrointestinal and hematologic malignances in patients with thrombocytopenia (clinicaltrails gov. Identifier: NCT02073682).
Figures
Comment in
-
Anticoagulation and thrombocytopenia in cancer: what more can we learn from existing randomized controlled trials.Haematologica. 2024 Jun 1;109(6):1648-1650. doi: 10.3324/haematol.2023.284291. Haematologica. 2024. PMID: 38105724 Free PMC article. No abstract available.
References
-
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore). 1999;78(5):285-291. - PubMed
-
- Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653-1661. - PubMed
-
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. - PubMed
-
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

