Diffuse traumatic brain injury substantially alters plasma growth hormone in the juvenile rat
- PMID: 37855319
- PMCID: PMC10692649
- DOI: 10.1530/JOE-23-0157
Diffuse traumatic brain injury substantially alters plasma growth hormone in the juvenile rat
Abstract
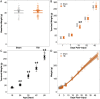
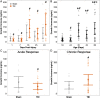
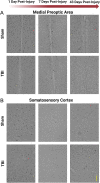
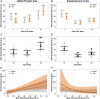
Traumatic brain injury (TBI) can damage the hypothalamus and cause improper activation of the growth hormone (GH) axis, leading to growth hormone deficiency (GHD). GHD is one of the most prevalent endocrinopathies following TBI in adults; however, the extent to which GHD affects juveniles remains understudied. We used postnatal day 17 rats (n = 83), which model the late infantile/toddler period, and assessed body weights, GH levels, and number of hypothalamic somatostatin neurons at acute (1, 7 days post injury (DPI)) and chronic (18, 25, 43 DPI) time points. We hypothesized that diffuse TBI would alter circulating GH levels because of damage to the hypothalamus, specifically somatostatin neurons. Data were analyzed with generalized linear and mixed effects models with fixed effects interactions between the injury and time. Despite similar growth rates over time with age, TBI rats weighed less than shams at 18 DPI (postnatal day 35; P = 0.03, standardized effect size [d] = 1.24), which is around the onset of puberty. Compared to shams, GH levels were lower in the TBI group during the acute period (P = 0.196; d = 12.3) but higher in the TBI group during the chronic period (P = 0.10; d = 52.1). Although not statistically significant, TBI-induced differences in GH had large standardized effect sizes, indicating biological significance. The mean number of hypothalamic somatostatin neurons (an inhibitor of GH) positively predicted GH levels in the hypothalamus but did not predict GH levels in the somatosensory cortex. Understanding TBI-induced alterations in the GH axis may identify therapeutic targets to improve the quality of life of pediatric survivors of TBI.
Keywords: concussion; development; growth hormone deficiency; pediatric; puberty; somatostatin.
Conflict of interest statement
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


Similar articles
-
Growth Hormone Deficiency Following Traumatic Brain Injury in Pediatric and Adolescent Patients: Presentation, Treatment, and Challenges of Transitioning from Pediatric to Adult Services.J Neurotrauma. 2023 Jul;40(13-14):1274-1285. doi: 10.1089/neu.2022.0384. Epub 2023 Mar 21. J Neurotrauma. 2023. PMID: 36825511 Free PMC article. Review.
-
Identifying Growth Hormone Deficiency in Brain-Injured Patients: The Quality of Life Scale-99.J Neurotrauma. 2025 Mar;42(5-6):379-390. doi: 10.1089/neu.2024.0114. Epub 2024 Dec 16. J Neurotrauma. 2025. PMID: 39681340
-
Growth Hormone Deficiency Following Traumatic Brain Injury.Int J Mol Sci. 2019 Jul 6;20(13):3323. doi: 10.3390/ijms20133323. Int J Mol Sci. 2019. PMID: 31284550 Free PMC article. Review.
-
Traumatic Brain Injury as Frequent Cause of Hypopituitarism and Growth Hormone Deficiency: Epidemiology, Diagnosis, and Treatment.Front Endocrinol (Lausanne). 2021 Mar 15;12:634415. doi: 10.3389/fendo.2021.634415. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33790864 Free PMC article. Review.
-
Deprivation of growth hormone-releasing hormone early in the rat's neonatal life permanently affects somatotropic function.Endocrinology. 1990 Oct;127(4):1625-34. doi: 10.1210/endo-127-4-1625. Endocrinology. 1990. PMID: 1976092
References
-
- Aimaretti G, Ambrosio MR, Benvenga S, Borretta G, De Marinis L, De Menis E, Di Somma C, Faustini-Fustini M, Grottoli S, Gasco V, et al.2004Hypopituitarism and growth hormone deficiency (GHD) after traumatic brain injury (TBI). Growth Hormone and IGF Research 14(Supplement A) S114–S117. (10.1016/j.ghir.2004.03.025) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

