Colorectal Cancer Screening for Persons With a Positive Family History—Evaluation of the FARKOR Program for the Secondary Prevention of Colorectal Cancer in Persons Aged 25 to 50
- PMID: 37855423
- PMCID: PMC10762841
- DOI: 10.3238/arztebl.m2023.0220
Colorectal Cancer Screening for Persons With a Positive Family History—Evaluation of the FARKOR Program for the Secondary Prevention of Colorectal Cancer in Persons Aged 25 to 50
Abstract
Background: Persons with a positive family history of colorectal cancer (CRC) are more likely than others to develop CRC and are also younger at the onset of the disease. Nonetheless, the German Federal Joint Committee (G-BA, Gemeinsamer Bundes - ausschuss) recommends screening all persons aged 50 and above regardless of their family history. FARKOR was a project supported by the Innovation Fund of the G-BA to study the feasibility, efficacy, and safety of a risk-adapted early detection program for CRC among persons aged 25 to 50 without any specific past medical history.
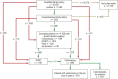
Methods: Physicians in private practice in Bavaria documented their activities relating to FARKOR online. The FARKOR process comprised a declaration of consent, a simplified family history for CRC, an optional, more comprehensive family history, a counseling session for participatory decision-making on further measures, and various modalities of screening (an immunological fecal occult blood test [iFOBT], colonoscopy, or no screening). Related physician activities outside the FARKOR process were assessed by record linkage between study data and data of the patients' health insurance carriers.
Results: The simplified family history was documented in 25 847 persons and positive for CRC in 5769 (22.3%). 3232 persons had a more comprehensive family history, among whom 2054 (63.6%) participated in screening measures. 1595 underwent colonoscopy; 278 persons who had already undergone colonoscopy in the preceding five years were excluded from the analysis. Colonoscopy revealed adenoma in 232 persons (17,6 %), advanced adenoma in 78 (5.9%) and carcinoma in 4 (0.3%). There were no serious complications.
Conclusion: The detection rates in this study corresponded to those of persons aged 55 to 59 in the current early detection program. Despite numerous problems in the performance of the study (inconsistencies in documentation, external performance of screening measures on program participants), the results support the feasibility of a risk-adapted early detection program in the young target population with a family history of CRC.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. - PubMed
-
- Gemeinsamer Bundesausschuss. Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krebserkrankungen (Krebsfrüherkennungs-Richtlinie/KFE-RL) www.g-ba.de/downloads/62-492-2238/KFE-RL_2020-06-18_iK-2020-08-28.pdf. (last accessed on 9 October 2023)
-
- Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

