In vivo-like nearest neighbor parameters improve prediction of fractional RNA base-pairing in cells
- PMID: 37855684
- PMCID: PMC10639048
- DOI: 10.1093/nar/gkad807
In vivo-like nearest neighbor parameters improve prediction of fractional RNA base-pairing in cells
Abstract
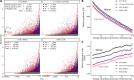
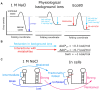
We conducted a thermodynamic analysis of RNA stability in Eco80 artificial cytoplasm, which mimics in vivo conditions, and compared it to transcriptome-wide probing of mRNA. Eco80 contains 80% of Escherichia coli metabolites, with biological concentrations of metal ions, including 2 mM free Mg2+ and 29 mM metabolite-chelated Mg2+. Fluorescence-detected binding isotherms (FDBI) were used to conduct a thermodynamic analysis of 24 RNA helices and found that these helices, which have an average stability of -12.3 kcal/mol, are less stable by ΔΔGo37 ∼1 kcal/mol. The FDBI data was used to determine a set of Watson-Crick free energy nearest neighbor parameters (NNPs), which revealed that Eco80 reduces the stability of three NNPs. This information was used to adjust the NN model using the RNAstructure package. The in vivo-like adjustments have minimal effects on the prediction of RNA secondary structures determined in vitro and in silico, but markedly improve prediction of fractional RNA base pairing in E. coli, as benchmarked with our in vivo DMS and EDC RNA chemical probing data. In summary, our thermodynamic and chemical probing analyses of RNA helices indicate that RNA secondary structures are less stable in cells than in artificially stable in vitro buffer conditions.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Cech T.R., Steitz J.A.. The noncoding RNA revolution—trashing old rules to forge new ones. Cell. 2014; 157:77–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

