Alfosbuvir plus Daclatasvir for Treatment of Chronic Hepatitis C Virus Infection in China
- PMID: 37856013
- PMCID: PMC10651614
- DOI: 10.1007/s40121-023-00872-4
Alfosbuvir plus Daclatasvir for Treatment of Chronic Hepatitis C Virus Infection in China
Abstract
Introduction: A pan-genotypic and effective treatment regimen for patients with chronic hepatitis C virus (HCV) infection remains an unmet medical need in China. Alfosbuvir is a novel potent HCV NS5B polymerase inhibitor in development for the treatment of chronic HCV infection. We conducted a phase 3 study to evaluate the efficacy and safety of alfosbuvir in combination with daclatasvir in Chinese patients with HCV infection.
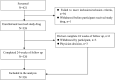
Methods: All patients received 600 mg alfosbuvir tablets plus 60 mg daclatasvir tablets once daily for 12 weeks. The primary endpoint was sustained virological response 12 weeks after the end of treatment (SVR12). A follow-up visit was done at week 4 and 12, and those who achieved SVR12 were followed up at post-treatment week 24.
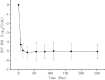
Results: Of the 326 patients who received at least one dose of the study drug, 320 (98.2% [95% confidence interval (CI): 96.5%-99.5%]) achieved sustained virological response at post-treatment week 12 (SVR12), which was superior to the historical SVR12 rate of 88% (p < 0.0001). The SVR12 rates were similar regardless of most baseline characteristics. The most common adverse event (AE) (≥ 10%) was hypercholesterolemia. Serious adverse events (SAEs) were reported in 25 (7.7%) patients, none of which was judged to be related to the study drug. The majority of AEs were mild to moderate in severity.
Conclusions: Alfosbuvir plus daclatasvir for 12 weeks was highly effective and safe in Chinese patients infected with HCV genotype 1, 2, 3, or 6, suggesting that this regimen could be a promising option for HCV treatment in China irrespective of genotype.
Trial registration: ClinicalTrial.gov identifier, NCT04070235.
Keywords: Alfosbuvir; China; Daclatasvir; Hepatitis C virus.
© 2023. The Author(s).
Conflict of interest statement
Xiaomeng Zhang, Jie Min, and Xinsheng Shi are employees of Nanjing Sanhome Pharmaceutical Co., Ltd. The other authors have no conflict of interest to declare. Rui Hua, Fei Kong, Guangming Li, Xiaofeng Wen, Yuexin Zhang, Xingxiang Yang, Chenxin Meng, Wen Xie, Yongfang Jiang, Xiaozhong Wang, Xueji Han, Yan Huang, Qing Mao, Jiefei Wang, Yujuan Guan, Jiayu Chen, Yingjie Ma, Qingfang Xion, Hong Ma, Xuebing Yan, Huiying Rao, Yingren Zhao, Tong Sun, Liying Zhu, Xiaorong Mao, Jianqi Lian, Guojiong Deng, Yongning Xin, Yifei Wang, Yinong Ye, Bin Xu, Hainv Gao, Youwen Tan, Dongliang Li, Dongliang Yang, Minghua Su, Junqi Niu, and Lai Wei have nothing to disclose.
Figures
Similar articles
-
Efficacy and safety of alfosbuvir plus daclatasvir in Chinese patients with hepatitis C virus genotypes 1, 2, 3, and 6 infection: An open-label, phase 2 study.J Viral Hepat. 2022 Jun;29(6):455-464. doi: 10.1111/jvh.13650. Epub 2022 Apr 8. J Viral Hepat. 2022. PMID: 35080256 Clinical Trial.
-
Safety and efficacy of an 8-week regimen of grazoprevir plus ruzasvir plus uprifosbuvir compared with grazoprevir plus elbasvir plus uprifosbuvir in participants without cirrhosis infected with hepatitis C virus genotypes 1, 2, or 3 (C-CREST-1 and C-CREST-2, part A): two randomised, phase 2, open-label trials.Lancet Gastroenterol Hepatol. 2017 Nov;2(11):805-813. doi: 10.1016/S2468-1253(17)30159-0. Epub 2017 Aug 10. Lancet Gastroenterol Hepatol. 2017. PMID: 28802816 Clinical Trial.
-
All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study.Hepatology. 2015 Apr;61(4):1127-35. doi: 10.1002/hep.27726. Epub 2015 Mar 10. Hepatology. 2015. PMID: 25614962 Free PMC article. Clinical Trial.
-
Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial.Lancet. 2015 Jun 20;385(9986):2502-9. doi: 10.1016/S0140-6736(15)60159-3. Epub 2015 Mar 31. Lancet. 2015. PMID: 25837829 Clinical Trial.
-
Daclatasvir: A Review in Chronic Hepatitis C.Drugs. 2016 Sep;76(14):1381-91. doi: 10.1007/s40265-016-0632-x. Drugs. 2016. PMID: 27550544 Review.
Cited by
-
Pathogen Detection in Spinal Infections: Next-Generation Sequencing Versus Conventional Microbiological Methods.Curr Med Sci. 2025 Apr;45(2):331-340. doi: 10.1007/s11596-025-00040-4. Epub 2025 Mar 26. Curr Med Sci. 2025. PMID: 40138143
-
Are there differences in efficacy and safety between local and imported direct-acting antiviral agents for hepatitis C in China?Infect Dis Poverty. 2025 Jul 25;14(1):75. doi: 10.1186/s40249-025-01344-2. Infect Dis Poverty. 2025. PMID: 40708038 Free PMC article.
-
Utilizing metagenomic next-generation sequencing for pathogen detection and diagnosis in lower respiratory tract infections in real-world clinical practice.Infection. 2024 Apr;52(2):625-636. doi: 10.1007/s15010-024-02185-1. Epub 2024 Feb 18. Infection. 2024. PMID: 38368306
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical