Targeted mutagenesis of flavonoid biosynthesis pathway genes reveals functional divergence in seed coat colour, oil content and fatty acid composition in Brassica napus L
- PMID: 37856327
- PMCID: PMC10826991
- DOI: 10.1111/pbi.14197
Targeted mutagenesis of flavonoid biosynthesis pathway genes reveals functional divergence in seed coat colour, oil content and fatty acid composition in Brassica napus L
Abstract
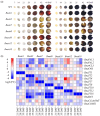
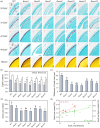
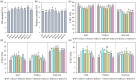
Yellow-seed is widely accepted as a good-quality trait in Brassica crops. Previous studies have shown that the flavonoid biosynthesis pathway is essential for the development of seed colour, but its function in Brassica napus, an important oil crop, is poorly understood. To systematically explore the gene functions of the flavonoid biosynthesis pathway in rapeseed, several representative TRANSPARENT TESTA (TT) genes, including three structural genes (BnaTT7, BnaTT18, BnaTT10), two regulatory genes (BnaTT1, BnaTT2) and a transporter (BnaTT12), were selected for targeted mutation by CRISPR/Cas9 in the present study. Seed coat colour, lignin content, seed quality and yield-related traits were investigated in these Bnatt mutants together with Bnatt8 generated previously. These Bnatt mutants produced seeds with an elevated seed oil content and decreased pigment and lignin accumulation in the seed coat without any serious defects in the yield-related traits. In addition, the fatty acid (FA) composition was also altered to different degrees, i.e., decreased oleic acid and increased linoleic acid and α-linolenic acid, in all Bnatt mutants except Bnatt18. Furthermore, gene expression analysis revealed that most of BnaTT mutations resulted in the down-regulation of key genes related to flavonoid and lignin synthesis, and the up-regulation of key genes related to lipid synthesis and oil body formation, which may contribute to the phenotype. Collectively, our study generated valuable resources for breeding programs, and more importantly demonstrated the functional divergence and overlap of flavonoid biosynthesis pathway genes in seed coat colour, oil content and FA composition of rapeseed.
Keywords: Brassica napus; CRISPR/Cas9; Fatty acid composition; Flavonoid biosynthesis pathway; Oil content; Seed coat colour.
© 2023 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures




Similar articles
-
Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L.Plant Biotechnol J. 2020 May;18(5):1153-1168. doi: 10.1111/pbi.13281. Epub 2019 Nov 11. Plant Biotechnol J. 2020. PMID: 31637846 Free PMC article.
-
Transcriptomic comparison between developing seeds of yellow- and black-seeded Brassica napus reveals that genes influence seed quality.BMC Plant Biol. 2019 May 16;19(1):203. doi: 10.1186/s12870-019-1821-z. BMC Plant Biol. 2019. PMID: 31096923 Free PMC article.
-
Targeted Knockout of BnTT2 Homologues for Yellow-Seeded Brassica napus with Reduced Flavonoids and Improved Fatty Acid Composition.J Agric Food Chem. 2020 May 20;68(20):5676-5690. doi: 10.1021/acs.jafc.0c01126. Epub 2020 May 12. J Agric Food Chem. 2020. PMID: 32394708
-
Molecular mechanism of manipulating seed coat coloration in oilseed Brassica species.J Appl Genet. 2013 May;54(2):135-45. doi: 10.1007/s13353-012-0132-y. Epub 2013 Jan 18. J Appl Genet. 2013. PMID: 23329015 Review.
-
The Flavonoid Biosynthesis and Regulation in Brassica napus: A Review.Int J Mol Sci. 2022 Dec 26;24(1):357. doi: 10.3390/ijms24010357. Int J Mol Sci. 2022. PMID: 36613800 Free PMC article. Review.
Cited by
-
Targeted mutation of BnaMS1/BnaMS2 combined with the RUBY reporter enables an efficient two-line system for hybrid seed production in Brassica napus.Hortic Res. 2024 Sep 25;12(1):uhae270. doi: 10.1093/hr/uhae270. eCollection 2025 Jan. Hortic Res. 2024. PMID: 39897731 Free PMC article.
-
Two Arabidopsis promoters drive seed-coat specific gene expression in pennycress and camelina.Plant Methods. 2023 Dec 6;19(1):140. doi: 10.1186/s13007-023-01114-x. Plant Methods. 2023. PMID: 38053155 Free PMC article.
-
The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition.Plants (Basel). 2025 Jan 13;14(2):202. doi: 10.3390/plants14020202. Plants (Basel). 2025. PMID: 39861556 Free PMC article.
-
CRISPR mutant rapid identification in B. napus: RNA-Seq functional profiling and breeding technology application.Front Plant Sci. 2025 Apr 22;16:1572020. doi: 10.3389/fpls.2025.1572020. eCollection 2025. Front Plant Sci. 2025. PMID: 40330131 Free PMC article.
-
Application and development of CRISPR technology in the secondary metabolic pathway of the active ingredients of phytopharmaceuticals.Front Plant Sci. 2025 Jan 9;15:1477894. doi: 10.3389/fpls.2024.1477894. eCollection 2024. Front Plant Sci. 2025. PMID: 39850214 Free PMC article. Review.
References
-
- Abbadi, A. and Leckband, G. (2011) Rapeseed breeding for oil content, quality, and sustainability. Eur. J. Lipid Sci. Tec. 113, 1198–1206.
-
- Abrahams, S. , Lee, E. , Walker, A.R. , Tanner, G.J. , Larkin, P.J. and Ashton, A.R. (2003) The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 35, 624–636. - PubMed
-
- Akhov, L. , Ashe, P. , Tan, Y. , Datla, R. and Selvaraj, G. (2009) Proanthocyanidin biosynthesis in the seed coat of yellow‐seeded, canola quality Brassica napus YN01‐429 is constrained at the committed step catalyzed by dihydroflavonol 4‐reductase. Botany 87, 616–625.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

