Associative and predictive hippocampal codes support memory-guided behaviors
- PMID: 37856604
- PMCID: PMC10894649
- DOI: 10.1126/science.adi8237
Associative and predictive hippocampal codes support memory-guided behaviors
Abstract
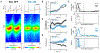
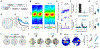
Episodic memory involves learning and recalling associations between items and their spatiotemporal context. Those memories can be further used to generate internal models of the world that enable predictions to be made. The mechanisms that support these associative and predictive aspects of memory are not yet understood. In this study, we used an optogenetic manipulation to perturb the sequential structure, but not global network dynamics, of place cells as rats traversed specific spatial trajectories. This perturbation abolished replay of those trajectories and the development of predictive representations, leading to impaired learning of new optimal trajectories during memory-guided navigation. However, place cell assembly reactivation and reward-context associative learning were unaffected. Our results show a mechanistic dissociation between two complementary hippocampal codes: an associative code (through coactivity) and a predictive code (through sequences).
Conflict of interest statement
Figures

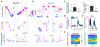

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

