Impact of follow ups, time interval and study duration in diffusion & myelin MRI clinical study in MS
- PMID: 37857232
- PMCID: PMC10591008
- DOI: 10.1016/j.nicl.2023.103529
Impact of follow ups, time interval and study duration in diffusion & myelin MRI clinical study in MS
Abstract
It is currently unknown how quantitative diffusion and myelin MRI designs affect the results of a longitudinal study. We used two independent datasets containing 6 monthly MRI measurements from 20 healthy controls and 20 relapsing-remitting multiple sclerosis (RR-MS) patients. Six designs were tested, including 3 MRI acquisitions, either over 6 months or over a shorter study duration, with balanced (same interval) or unbalanced (different interval) time intervals between MRI acquisitions. First, we show that in RR-MS patients, the brain changes over time obtained with 3 MRI acquisitions were similar to those observed with 5 MRI acquisitions and that designs with an unbalanced time interval showed the highest similarity, regardless of study duration. No significant brain changes were found in the healthy controls over the same periods. Second, the study duration affects the sample size in the RR-MS dataset; a longer study requires more subjects and vice versa. Third, the number of follow-up acquisitions and study duration affect the sensitivity and specificity of the associations with clinical parameters, and these depend on the white matter bundle and MRI measure considered. Together, this suggests that the optimal design depends on the assumption of the dynamics of change in the target population and the accuracy required to capture these dynamics. Thus, this work provides a better understanding of key factors to consider in a longitudinal study and provides clues for better strategies in clinical trial design.
Keywords: Diffusion MRI; Follow-ups; Healthy controls; Inhomogeneous magnetization transfer; Longitudinal study; Multiple Sclerosis; Study duration; Time-interval.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
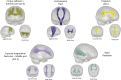

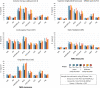

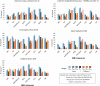

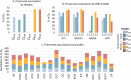
References
-
- Beare R., Ball G., Yang J.-Y.-M., Moran C., Srikanth V., Seal M. Participant followup rate can bias structural imaging measures in longitudinal studies. Neuroimage: Reports. 2021;1 doi: 10.1016/j.ynirp.2021.100066. - DOI
-
- Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

