Vaccination with post-translational modified, homocitrullinated peptides induces CD8 T-cell responses that mediate antitumor immunity
- PMID: 37857526
- PMCID: PMC10603355
- DOI: 10.1136/jitc-2023-006966
Vaccination with post-translational modified, homocitrullinated peptides induces CD8 T-cell responses that mediate antitumor immunity
Abstract
Background: Post-translational modification of proteins has the potential to alter the ability of T cells to recognize major histocompatibility complex (MHC) class -I and class-II restricted antigens, thereby resulting in altered immune responses. One such modification is carbamylation (homocitrullination) that results in the formation of homocitrulline (Hcit) residues in a non-enzymatic reaction of cyanate with the lysine residues in the polypeptide chain. Homocitrullination occurs in the tumor microenvironment and CD4-mediated immune responses to Hcit epitopes can target stressed tumor cells and provide a potent antitumor response in mouse models.
Methods: Homocitrullinated peptides were identified and assessed in vitro for HLA-A2 binding and in vivo in human leukocyte antigen (HLA) transgenic mouse models for immunogenicity. CD8 responses were assessed in vitro for cytotoxicity and in vivo tumor therapy. Human tumor samples were analyzed by targeted mass spectrometry for presence of homocitrullinated peptides.
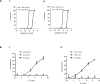
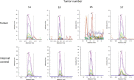
Results: Homocitrullinated peptides from aldolase and cytokeratin were identified, that stimulated CD8-mediated responses in vivo. Modified peptides showed enhanced binding to HLA-A2 compared with the native sequences and immunization of HLA-A2 transgenic mice generated high avidity modification specific CD8 responses that killed peptide expressing target cells. Importantly, in vivo the homocitrullinated aldolase specific response was associated with efficient CD8 dependent antitumor therapy of the aggressive murine B16 tumor model indicating that this epitope is naturally presented in the tumor. In addition, the homocitrullinated aldolase epitope was also detected in human tumor samples.
Conclusion: This is the first evidence that homocitrullinated peptides can be processed and presented via MHC-I and targeted for tumor therapy. Thus, Hcit-specific CD8 T-cell responses have potential in the development of future anticancer therapy.
Keywords: CD8-positive T-lymphocytes; antigens; immunogenicity, vaccine; immunotherapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KWC, VAB and LGD have ownership interests in a patent. LGD is a director and shareholder in Scancell Ltd. SS, KWC, PS, AS, AAO, SJP and VAB are employees of Scancell Ltd. JW and IP are employees of Proteome Sciences.
Figures




Similar articles
-
Homocitrullination of lysine residues mediated by myeloid-derived suppressor cells in the tumor environment is a target for cancer immunotherapy.J Immunother Cancer. 2021 Jul;9(7):e001910. doi: 10.1136/jitc-2020-001910. J Immunother Cancer. 2021. PMID: 34321274 Free PMC article.
-
Vaccine Can Induce CD4-Mediated Responses to Homocitrullinated Peptides via Multiple HLA-Types and Confer Anti-Tumor Immunity.Front Immunol. 2022 Apr 8;13:873947. doi: 10.3389/fimmu.2022.873947. eCollection 2022. Front Immunol. 2022. PMID: 35464453 Free PMC article.
-
Identification of human MHC-I HPV18 E6/E7-specific CD8 + T cell epitopes and generation of an HPV18 E6/E7-expressing adenosquamous carcinoma in HLA-A2 transgenic mice.J Biomed Sci. 2022 Oct 12;29(1):80. doi: 10.1186/s12929-022-00864-5. J Biomed Sci. 2022. PMID: 36224625 Free PMC article.
-
[MHC tetramers: tracking specific immunity].Acta Med Croatica. 2003;57(4):255-9. Acta Med Croatica. 2003. PMID: 14639858 Review. Croatian.
-
Towards Clinical Translation of CD8+ Regulatory T Cells Restricted by Non-Classical Major Histocompatibility Complex Ib Molecules.Int J Mol Sci. 2019 Sep 28;20(19):4829. doi: 10.3390/ijms20194829. Int J Mol Sci. 2019. PMID: 31569411 Free PMC article. Review.
Cited by
-
Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells.Vaccines (Basel). 2025 Jun 11;13(6):629. doi: 10.3390/vaccines13060629. Vaccines (Basel). 2025. PMID: 40573960 Free PMC article.
-
Protein carbamylation and proteomics: from artifacts to elucidation of biological functions.Front Anal Sci. 2024;4:1512573. doi: 10.3389/frans.2024.1512573. Epub 2025 Jan 2. Front Anal Sci. 2024. PMID: 40855863 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
