Efficacy of Tuina in chronic low back pain with anxiety: study protocol for a randomised controlled trial
- PMID: 37857544
- PMCID: PMC10603401
- DOI: 10.1136/bmjopen-2023-073671
Efficacy of Tuina in chronic low back pain with anxiety: study protocol for a randomised controlled trial
Abstract
Introduction: Chronic low back pain (cLBP) is one of the largest and most frequent public health problems worldwide. Tuina is a physical therapy commonly used in China to treat musculoskeletal diseases. Compared with traction, there is little high-quality scientific evidence that can demonstrate the effectiveness of Tuina in the treatment of patients with cLBP. Therefore, the purpose of this clinical trial is to evaluate the effect of massage on cLBP patients compared with traction.
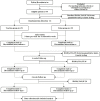
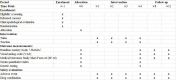
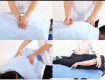
Methods and analyses: This is a single-centre, assessor-blinded and analyst-blinded prospective randomised controlled trial with two parallel arms. Ninety-four patients with cLBP will be recruited. Three treatments were given every week for a total of 4 weeks. In the Traction group, participants were given traction therapy in the Tuina group, participants will receive a four-step physiotherapy including kneading, rolling, plucking and oblique pulling. The outcomes will be measured at baseline, at the end of treatment, as well as 1 and 2 months after treatment. The primary outcome will be the Hamilton Anxiety Scale after 12 sessions of treatment. The secondary outcomes will be the Visual Analogue Scale, the medical outcomes study Short Form 36, Serum Quantitative Index and genetic testing after 12 sessions of treatment.
Ethics and dissemination: The study was approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated with Shanghai University of Traditional Chinese Medicine.
Trial registration number: ChiCTR2200065448.
Keywords: back pain; chronic pain; complementary medicine.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Xie Z, Bai J, Jian G, et al. Research progress on traditional Chinese and Western medicine in the treatment of lumbar disc herniation. Chinese Medicine Modern Distance Education of China 2021;19:196–8. 10.25236/IJFM.2022.041108 - DOI
-
- Fu Y, Zhang H, Zhang B, et al. Clinical observation of therapeutic methods of different sensitive types for lumbar disc herniation. Zhongguo Zhen Jiu 2015;35:1253–7. - PubMed
-
- Kim Y-K, Kang D, Lee I, et al. Differences in the incidence of symptomatic cervical and lumbar disc herniation according to age, sex and national health insurance eligibility: a pilot study on the disease's association with work. Int J Environ Res Public Health 2018;15:2094. 10.3390/ijerph15102094 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
