Epidemiology of Plasmodium malariae and Plasmodium ovale spp. in Kinshasa Province, Democratic Republic of Congo
- PMID: 37857597
- PMCID: PMC10587068
- DOI: 10.1038/s41467-023-42190-w
Epidemiology of Plasmodium malariae and Plasmodium ovale spp. in Kinshasa Province, Democratic Republic of Congo
Abstract
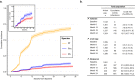
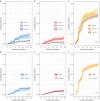
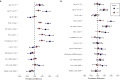
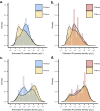
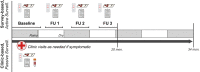
Reports suggest non-falciparum species are an underappreciated cause of malaria in sub-Saharan Africa but their epidemiology is ill-defined, particularly in highly malaria-endemic regions. We estimated incidence and prevalence of PCR-confirmed non-falciparum and Plasmodium falciparum malaria infections within a longitudinal study conducted in Kinshasa, Democratic Republic of Congo (DRC) between 2015-2017. Children and adults were sampled at biannual household surveys and routine clinic visits. Among 9,089 samples from 1,565 participants, incidences of P. malariae, P. ovale spp., and P. falciparum infections by 1-year were 7.8% (95% CI: 6.4%-9.1%), 4.8% (95% CI: 3.7%-5.9%) and 57.5% (95% CI: 54.4%-60.5%), respectively. Non-falciparum prevalences were higher in school-age children, rural and peri-urban sites, and P. falciparum co-infections. P. falciparum remains the primary driver of malaria in the DRC, though non-falciparum species also pose an infection risk. As P. falciparum interventions gain traction in high-burden settings, continued surveillance and improved understanding of non-falciparum infections are warranted.
© 2023. Springer Nature Limited.
Conflict of interest statement
J.B.P. reports research support from Gilead Sciences, non-financial support from Abbott Laboratories, and consulting for Zymeron Corporation, all outside the scope of this study. The remaining authors declare no competing interests.
Figures
Update of
-
Epidemiology of Plasmodium malariae and Plasmodium ovale spp. in a highly malaria-endemic country: a longitudinal cohort study in Kinshasa Province, Democratic Republic of Congo.medRxiv [Preprint]. 2023 Apr 25:2023.04.20.23288826. doi: 10.1101/2023.04.20.23288826. medRxiv. 2023. Update in: Nat Commun. 2023 Oct 19;14(1):6618. doi: 10.1038/s41467-023-42190-w. PMID: 37790376 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. World Malaria Report 2021. Genève, Switzerland; 2021. Accessed at https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
-
- Oboh MA, et al. Rising report of Plasmodium vivax in sub-Saharan Africa: implications for malaria elimination agenda. Sci. Afr. 2020;10:e00596.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

