Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456
- PMID: 37857609
- PMCID: PMC10587311
- DOI: 10.1038/s41392-023-01660-9
Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456
Abstract
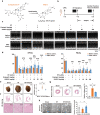
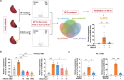
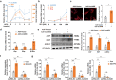
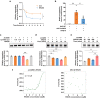
Reversing ventricular remodeling represents a promising treatment for the post-myocardial infarction (MI) heart failure (HF). Here, we report a novel small molecule HHQ16, an optimized derivative of astragaloside IV, which effectively reversed infarction-induced myocardial remodeling and improved cardiac function by directly acting on the cardiomyocyte to reverse hypertrophy. The effect of HHQ16 was associated with a strong inhibition of a newly discovered Egr2-affiliated transcript lnc9456 in the heart. While minimally expressed in normal mouse heart, lnc9456 was dramatically upregulated in the heart subjected to left anterior descending coronary artery ligation (LADL) and in cardiomyocytes subjected to hypertrophic stimulation. The critical role of lnc9456 in cardiomyocyte hypertrophy was confirmed by specific overexpression and knockout in vitro. A physical interaction between lnc9456 and G3BP2 increased NF-κB nuclear translocation, triggering hypertrophy-related cascades. HHQ16 physically bound to lnc9456 with a high-affinity and induced its degradation. Cardiomyocyte-specific lnc9456 overexpression induced, but knockout prevented LADL-induced, cardiac hypertrophy and dysfunction. HHQ16 reversed the effect of lnc9456 overexpression while lost its protective role when lnc9456 was deleted, further confirming lnc9456 as the bona fide target of HHQ16. We further identified the human ortholog of lnc9456, also an Egr2-affiliated transcript, lnc4012. Similarly, lnc4012 was significantly upregulated in hypertrophied failing hearts of patients with dilated cardiomyopathy. HHQ16 also specifically bound to lnc4012 and caused its degradation and antagonized its hypertrophic effects. Targeted degradation of pathological increased lnc4012/lnc9456 by small molecules might serve as a novel promising strategy to regress infarction-induced cardiac hypertrophy and HF.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

