Utility of nasal swabs for assessing mucosal immune responses towards SARS-CoV-2
- PMID: 37857783
- PMCID: PMC10587113
- DOI: 10.1038/s41598-023-44989-5
Utility of nasal swabs for assessing mucosal immune responses towards SARS-CoV-2
Abstract
SARS-CoV-2 has caused millions of infections worldwide since its emergence in 2019. Understanding how infection and vaccination induce mucosal immune responses and how they fluctuate over time is important, especially since they are key in preventing infection and reducing disease severity. We established a novel methodology for assessing SARS-CoV-2 cytokine and antibody responses at the nasal epithelium by using nasopharyngeal swabs collected longitudinally before and after either SARS-CoV-2 infection or vaccination. We then compared responses between mucosal and systemic compartments. We demonstrate that cytokine and antibody profiles differ between compartments. Nasal cytokines show a wound healing phenotype while plasma cytokines are consistent with pro-inflammatory pathways. We found that nasal IgA and IgG have different kinetics after infection, with IgA peaking first. Although vaccination results in low nasal IgA, IgG induction persists for up to 180 days post-vaccination. This research highlights the importance of studying mucosal responses in addition to systemic responses to respiratory infections. The methods described herein can be used to further mucosal vaccine development by giving us a better understanding of immunity at the nasal epithelium providing a simpler, alternative clinical practice to studying mucosal responses to infection.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
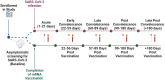

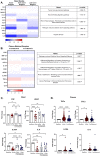
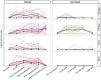


Update of
-
Utility of nasal swabs for assessing mucosal immune responses towards SARS-CoV-2.bioRxiv [Preprint]. 2023 Jul 13:2023.07.12.548630. doi: 10.1101/2023.07.12.548630. bioRxiv. 2023. Update in: Sci Rep. 2023 Oct 19;13(1):17820. doi: 10.1038/s41598-023-44989-5. PMID: 37503213 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

