Molecular hybridization strategy for tuning bioactive peptide function
- PMID: 37857855
- PMCID: PMC10587126
- DOI: 10.1038/s42003-023-05254-7
Molecular hybridization strategy for tuning bioactive peptide function
Abstract
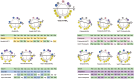
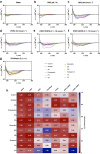
The physicochemical and structural properties of antimicrobial peptides (AMPs) determine their mechanism of action and biological function. However, the development of AMPs as therapeutic drugs has been traditionally limited by their toxicity for human cells. Tuning the physicochemical properties of such molecules may abolish toxicity and yield synthetic molecules displaying optimal safety profiles and enhanced antimicrobial activity. Here, natural peptides were modified to improve their activity by the hybridization of sequences from two different active peptide sequences. Hybrid AMPs (hAMPs) were generated by combining the amphipathic faces of the highly toxic peptide VmCT1, derived from scorpion venom, with parts of four other naturally occurring peptides having high antimicrobial activity and low toxicity against human cells. This strategy led to the design of seven synthetic bioactive variants, all of which preserved their structure and presented increased antimicrobial activity (3.1-128 μmol L-1). Five of the peptides (three being hAMPs) presented high antiplasmodial at 0.8 μmol L-1, and virtually no undesired toxic effects against red blood cells. In sum, we demonstrate that peptide hybridization is an effective strategy for redirecting biological activity to generate novel bioactive molecules with desired properties.
© 2023. Springer Nature Limited.
Conflict of interest statement
C.F.N. provides consulting services to Invaio Sciences and is a member of the Scientific Advisory Boards of Nowture S.L. and Phare Bio. The de la Fuente Lab has received research funding or in-kind donations from United Therapeutics, Strata Manufacturing PJSC, and Procter & Gamble for research unrelated to that presented here. A provisional patent application has been filed on the de la Fuente Lab’s related work (ID number 23-10378). All other authors declare no competing interests.
Figures




References
-
- Tacconelli E, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. - PubMed
-
- World Health Organization. WHO World Malaria Report 2020. Malaria report 2020 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

