Molecular Phenotyping and Mechanisms of Myocardial Fibrosis in Advanced Chronic Kidney Disease
- PMID: 37858297
- PMCID: PMC10695648
- DOI: 10.34067/KID.0000000000000276
Molecular Phenotyping and Mechanisms of Myocardial Fibrosis in Advanced Chronic Kidney Disease
Abstract
Key Points:
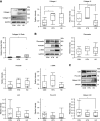
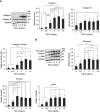
Myocardial fibrosis in hearts from patients with CKD is characterized by increased trimeric tensile collagen type I and decreased elastic collagen type III compared with hearts from hypertensive or healthy donors, suggesting a unique fibrotic phenotype.
Myocardial fibrosis in CKD is driven by alterations in extracellular matrix proteostasis, including dysregulation of metalloproteinases and cross-linking enzymes.
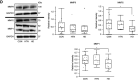
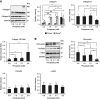
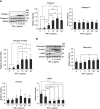
CKD-associated mineral stressors uniquely induce a fibronectin-independent mechanism of fibrillogenesis characterized by formation of trimeric collagen compared with proinflammatory/fibrotic cytokines.
Background: Myocardial fibrosis is a major life-limiting problem in CKD. Despite this, the molecular phenotype and metabolism of collagen fibrillogenesis in fibrotic hearts of patients with advanced CKD have been largely unstudied.
Methods: We analyzed explanted human left ventricular (LV) heart tissues in a three-arm cross-sectional cohort study of deceased donor patients on hemodialysis (HD, n=18), hypertension with preserved renal function (HTN, n=8), and healthy controls (CON, n=17), ex vivo. RNA-seq and protein analysis was performed on human donor hearts and cardiac fibroblasts treated with mineral stressors (high phosphate and high calcium). Further mechanistic studies were performed using primary cardiac fibroblasts, in vitro treated with mineral stressors, proinflammatory and profibrotic cytokines.
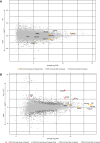
Results: Of the 43 donor participants, there was no difference in age (P > 0.2), sex (P > 0.8), or body mass index (P > 0.1) between the groups. Hearts from the HD group had extensive fibrosis (P < 0.01). All LV tissues expressed only the trimeric form of collagen type I. HD hearts expressed increased collagen type I (P < 0.03), elevated collagen type I:III ratio (P < 0.05), and decreased MMP1 (P < 0.05) and MMP2 (P < 0.05). RNA-seq revealed no significant differential gene expression of extracellular matrix proteins of interest in HD hearts, but there was significant upregulation of LH2, periostin, α-SMA, and TGF-β1 gene expression in mineral stressor–treated cardiac fibroblasts. Both mineral stressors (P < 0.009) and cytokines (P < 0.03) increased collagen type I:III ratio. Mineral stressors induced trimeric collagen type I, but cytokine treatment induced only dimeric collagen type I in cardiac fibroblasts. Mineral stressors downregulated fibronectin (P < 0.03) and MMP2 zymogen (P < 0.01) but did not significantly affect expression of periostin, MMP1, or cross-linking enzymes. TGF-β upregulated fibronectin (P < 0.01) and periostin (P < 0.02) only.
Conclusions: Myocardial fibrosis in advanced CKD hearts is characterized by increased trimeric collagen type I and dysregulated collagen metabolism, and is differentially regulated by components of uremia.
Conflict of interest statement
K. Lim reports consultancy agreements with Ambassadors Global and MBX Biosciences; ownership interest in OVIBIO Corporation, Ambassadors Global, and MBX Biosciences; advisory or leadership role in Ambassadors Global MBX Biosciences; and PI grant of Dialysis Clinics Inc (DCI) (Paul Teschan Research Fund (PTRF)), IU Health Values Fund Award. T. Lu reports ownership interest in Ovibio corporation, and CHF Foundation Funding Award. S. Moe reports consultancy agreements with Amgen, Ardelyx, Sanifit/Vifor, and Inozyme; ownership interest in Eli Lilly (stock); research funding from NIH-research grant and Keryx-research grant; honoraria from Ardelyx, Sanifit, and Inozyme; and advisory or leadership role in Editorial Board of AJ Nephrology and AJ Nutrition. G. Narayanan reports patents or royalties from Indiana University. A. Halim reports NIH NRSA Fellowship from NIH T32 AR065971, ownership interest in OVIBIO Corporation and patents or royalties from Indiana University. D. Zehnder reports research funding from Abbott Laboratories and Amgen and honoraria from Abbott Laboratories and Amgen. The remaining authors have nothing to disclose.
Figures




Comment in
-
Insights into Myocardial Fibrosis in Advanced Chronic Kidney Disease Using Human Tissue.Kidney360. 2023 Nov 1;4(11):1531-1533. doi: 10.34067/KID.0000000000000285. Kidney360. 2023. PMID: 38032767 Free PMC article. No abstract available.
References
-
- Edwards NC, Hayer MK, Ferro CJ, Townend JN, Steeds RP. CKD associated cardiomyopathy: molecular mechanisms, imaging modalities, disease evolution and interventions. In: Rangaswami J, Lerma EV, Ronco C, eds. Cardio-Nephrology: Confluence of the Heart and Kidney in Clinical Practice. Springer International Publishing; 2017:45–58.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

