The microtubule-severing protein UNC-45A preferentially binds to curved microtubules and counteracts the microtubule-straightening effects of Taxol
- PMID: 37858676
- PMCID: PMC10654038
- DOI: 10.1016/j.jbc.2023.105355
The microtubule-severing protein UNC-45A preferentially binds to curved microtubules and counteracts the microtubule-straightening effects of Taxol
Abstract
Uncoordinated protein 45A (UNC-45A) is the only known ATP-independent microtubule (MT)-severing protein. Thus, it severs MTs via a novel mechanism. In vitro and in cells, UNC-45A-mediated MT severing is preceded by the appearance of MT bends. While MTs are stiff biological polymers, in cells, they often curve, and the result of this curving can be breaking off. The contribution of MT-severing proteins on MT lattice curvature is largely undefined. Here, we show that UNC-45A curves MTs. Using in vitro biophysical reconstitution and total internal fluorescence microscopy analysis, we show that UNC-45A is enriched in the areas where MTs are curved versus the areas where MTs are straight. In cells, we show that UNC-45A overexpression increases MT curvature and its depletion has the opposite effect. We also show that this effect occurs is independent of actomyosin contractility. Lastly, we show for the first time that in cells, Paclitaxel straightens MTs, and that UNC-45A can counteracts the MT-straightening effects of the drug.
Keywords: UNC-45A; microtubule curvature; microtubules.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


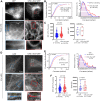




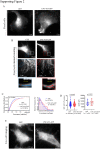


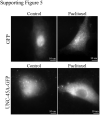
Update of
-
The Microtubule Severing Protein UNC-45A Counteracts the Microtubule Straightening Effects of Taxol.bioRxiv [Preprint]. 2023 Sep 13:2023.09.12.557417. doi: 10.1101/2023.09.12.557417. bioRxiv. 2023. Update in: J Biol Chem. 2023 Nov;299(11):105355. doi: 10.1016/j.jbc.2023.105355. PMID: 37745537 Free PMC article. Updated. Preprint.
References
-
- Felgner H., Frank R., Schliwa M. Flexural rigidity of microtubules measured with the use of optical tweezers. J. Cell Sci. 1996;109:509–516. - PubMed
-
- Bicek A.D., Tüzel E., Kroll D.M., Odde D.J. Analysis of microtubule curvature. Methods Cell Biol. 2007;83:237–268. - PubMed
-
- Odde D.J., Ma L., Briggs A.H., DeMarco A., Kirschner M.W. Microtubule bending and breaking in living fibroblast cells. J. Cell Sci. 1999;112:3283–3288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

