Ghrelin delays premature aging in Hutchinson-Gilford progeria syndrome
- PMID: 37858983
- PMCID: PMC10726901
- DOI: 10.1111/acel.13983
Ghrelin delays premature aging in Hutchinson-Gilford progeria syndrome
Abstract
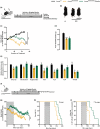
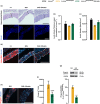
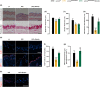
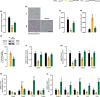
Hutchinson-Gilford progeria syndrome (HGPS) is a rare and fatal genetic condition that arises from a single nucleotide alteration in the LMNA gene, leading to the production of a defective lamin A protein known as progerin. The accumulation of progerin accelerates the onset of a dramatic premature aging phenotype in children with HGPS, characterized by low body weight, lipodystrophy, metabolic dysfunction, skin, and musculoskeletal age-related dysfunctions. In most cases, these children die of age-related cardiovascular dysfunction by their early teenage years. The absence of effective treatments for HGPS underscores the critical need to explore novel safe therapeutic strategies. In this study, we show that treatment with the hormone ghrelin increases autophagy, decreases progerin levels, and alleviates other cellular hallmarks of premature aging in human HGPS fibroblasts. Additionally, using a HGPS mouse model (LmnaG609G/G609G mice), we demonstrate that ghrelin administration effectively rescues molecular and histopathological progeroid features, prevents progressive weight loss in later stages, reverses the lipodystrophic phenotype, and extends lifespan of these short-lived mice. Therefore, our findings uncover the potential of modulating ghrelin signaling offers new treatment targets and translational approaches that may improve outcomes and enhance the quality of life for patients with HGPS and other age-related pathologies.
Keywords: autophagy; ghrelin; human aging; progeria; senescence.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


References
-
- Akamizu, T. , Murayama, T. , Teramukai, S. , Miura, K. , Bando, I. , Irako, T. , Iwakura, H. , Ariyasu, H. , Hosoda, H. , Tada, H. , Matsuyama, A. , Kojima, S. , Wada, T. , Wakatsuki, Y. , Matsubayashi, K. , Kawakita, T. , Shimizu, A. , Fukushima, M. , Yokode, M. , & Kangawa, K. (2006). Plasma ghrelin levels in healthy elderly volunteers: The levels of acylated ghrelin in elderly females correlate positively with serum IGF‐I levels and bowel movement frequency and negatively with systolic blood pressure. The Journal of Endocrinology, 188(2), 333–344. 10.1677/joe.1.06442 - DOI - PubMed
-
- Aveleira, C. A. , Ferreira‐Marques, M. , Cortes, L. , Valero, J. , Pereira, D. , Pereira de Almeida, L. , & Cavadas, C. (2020). Neuropeptide Y enhances Progerin clearance and ameliorates the senescent phenotype of human Hutchinson‐Gilford progeria syndrome cells. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(6), 1073–1078. 10.1093/gerona/glz280 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

