A Monocytic Barrier to the Humanization of Immunodeficient Mice
- PMID: 37859310
- PMCID: PMC10997744
- DOI: 10.2174/011574888X263597231001164351
A Monocytic Barrier to the Humanization of Immunodeficient Mice
Abstract
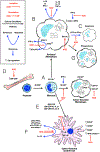
Mice with severe immunodeficiencies have become very important tools for studying foreign cells in an in vivo environment. Xenotransplants can be used to model cells from many species, although most often, mice are humanized through the transplantation of human cells or tissues to meet the needs of medical research. The development of immunodeficient mice is reviewed leading up to the current state-of-the-art strains, such as the NOD-scid-gamma (NSG) mouse. NSG mice are excellent hosts for human hematopoietic stem cell transplants or immune reconstitution through transfusion of human peripheral blood mononuclear cells. However, barriers to full hematopoietic engraftment still remain; notably, the survival of human cells in the circulation is brief, which limits overall hematological and immune reconstitution. Reports have indicated a critical role for monocytic cells - monocytes, macrophages, and dendritic cells - in the clearance of xenogeneic cells from circulation. Various aspects of the NOD genetic background that affect monocytic cell growth, maturation, and function that are favorable to human cell transplantation are discussed. Important receptors, such as SIRPα, that form a part of the innate immune system and enable the recognition and phagocytosis of foreign cells by monocytic cells are reviewed. The development of humanized mouse models has taken decades of work in creating more immunodeficient mice, genetic modification of these mice to express human genes, and refinement of transplant techniques to optimize engraftment. Future advances may focus on the monocytic cells of the host to find ways for further engraftment and survival of xenogeneic cells.
Keywords: Mice; SCID; blood cells; dendritic cells; humanized mice; macrophages; myeloid cells; transplantation..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
CONFLICT OF INTEREST
Dr. Marcus Muench is on the editorial advisory board of the journal CSCRT.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

