PRMT1 accelerates cell proliferation, migration, and tumor growth by upregulating ZEB1/H4R3me2as in thyroid carcinoma
- PMID: 37859611
- PMCID: PMC10603553
- DOI: 10.3892/or.2023.8647
PRMT1 accelerates cell proliferation, migration, and tumor growth by upregulating ZEB1/H4R3me2as in thyroid carcinoma
Abstract
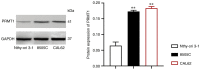
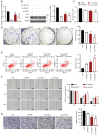
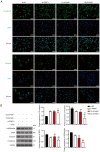
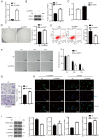
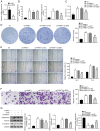
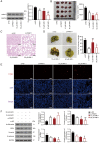
Thyroid carcinoma (TC) represents the most prevalent malignancy of the endocrine system. Protein arginine methyltransferase 1 (PRMT1) is a critical member of the protein arginine methyltransferase family in mammals and is involved in multiple biological processes. This study aimed to investigate the function of PRMT1 in TC. In the present study, human TC cell lines (8505C, CAL62, and BCPAP) and a normal human thyroid cell line Nthy‑ori 3‑1 were employed. Small interfering RNA targeting PRMT1 was used to knock down PRMT1 expression in 8505C cells, and PRMT1 overexpression plasmids were transfected into BCPAP cells. Cell viability was assessed using a CCK‑8 and colony formation assays. Apoptosis was measured using flow cytometry and TUNEL assays. Cell migration was assessed using wound healing and Transwell assays. Reverse transcription‑quantitative PCR was used to determine the mRNA expression levels of PRMT1. Western blotting was used to detect the protein expression levels of PRMT1, E‑cadherin, vimentin, H4R3me2as, and zinc‑finger E homeobox‑binding 1 (ZEB1). Notably, PRMT1 expression was elevated in TC (P<0.01). PRMT1 knockdown inhibited TC cell proliferation and migration and concurrently enhanced migration. Furthermore, PRMT1 knockdown suppressed tumor growth and metastasis in a mouse model of TC. PRMT1 downregulation increased E‑cadherin expression and decreased the expression of vimentin, H4R3me2as, and ZEB1 in the TC cells and the mouse model of TC. Conversely, PRMT1 overexpression had the opposite effect on TC malignant characteristics (P<0.05). These findings suggest that PRMT1 knockdown inhibited TC malignancy by downregulating H4R3me2as/ZEB1, thereby highlighting novel therapeutic targets and diagnostic markers for the management of TC.
Keywords: H4R3me2; ZEB1; malignancy; protein arginine methyltransferase 1; thyroid carcinoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1.Sci Rep. 2016 Jan 27;6:19874. doi: 10.1038/srep19874. Sci Rep. 2016. PMID: 26813495 Free PMC article.
-
Overexpressing PRMT1 Inhibits Proliferation and Invasion in Pancreatic Cancer by Inverse Correlation of ZEB1.IUBMB Life. 2018 Oct;70(10):1032-1039. doi: 10.1002/iub.1917. Epub 2018 Sep 8. IUBMB Life. 2018. PMID: 30194893
-
UBE2C, Directly Targeted by miR-548e-5p, Increases the Cellular Growth and Invasive Abilities of Cancer Cells Interacting with the EMT Marker Protein Zinc Finger E-box Binding Homeobox 1/2 in NSCLC.Theranostics. 2019 Mar 17;9(7):2036-2055. doi: 10.7150/thno.32738. eCollection 2019. Theranostics. 2019. Retraction in: Theranostics. 2020 Jul 25;10(21):9619. doi: 10.7150/thno.50254. PMID: 31037155 Free PMC article. Retracted.
-
MicroRNA‑873 inhibits the progression of thyroid cancer by directly targeting ZEB1.Mol Med Rep. 2019 Aug;20(2):1986-1993. doi: 10.3892/mmr.2019.10381. Epub 2019 Jun 12. Mol Med Rep. 2019. PMID: 31257462
-
Role of ZEB Family Members in Proliferation, Metastasis, and Chemoresistance of Prostate Cancer Cells: Revealing Signaling Networks.Curr Cancer Drug Targets. 2021;21(9):749-767. doi: 10.2174/1568009621666210601114631. Curr Cancer Drug Targets. 2021. PMID: 34077345 Review.
Cited by
-
Strategic targeting of miR-183 and β-catenin to enhance BMSC stemness in age-related osteoporosis therapy.Sci Rep. 2024 Sep 14;14(1):21489. doi: 10.1038/s41598-024-72474-0. Sci Rep. 2024. PMID: 39277663 Free PMC article.
-
FN1, a reliable prognostic biomarker for thyroid cancer, is associated with tumor immunity and an unfavorable prognosis.Oncol Lett. 2024 Aug 26;28(5):510. doi: 10.3892/ol.2024.14643. eCollection 2024 Nov. Oncol Lett. 2024. PMID: 39268167 Free PMC article.
-
Expression of CLDN1 and EGFR in PTC.Discov Oncol. 2024 Oct 15;15(1):562. doi: 10.1007/s12672-024-01428-9. Discov Oncol. 2024. PMID: 39404969 Free PMC article.
-
PRMT1-mediated modification of H4R3me2a promotes liver cancer progression by enhancing the transcriptional activity of SOX18.Hepatol Commun. 2025 Mar 24;9(4):e0647. doi: 10.1097/HC9.0000000000000647. eCollection 2025 Apr 1. Hepatol Commun. 2025. PMID: 40130992 Free PMC article.
-
Oligomerization of protein arginine methyltransferase 1 and its effect on methyltransferase activity and substrate specificity.Protein Sci. 2024 Aug;33(8):e5118. doi: 10.1002/pro.5118. Protein Sci. 2024. PMID: 39022984 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

