Polycystic ovary syndrome: pathophysiology and therapeutic opportunities
- PMID: 37859784
- PMCID: PMC10583117
- DOI: 10.1136/bmjmed-2023-000548
Polycystic ovary syndrome: pathophysiology and therapeutic opportunities
Abstract
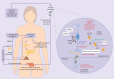
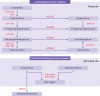
Polycystic ovary syndrome is characterised by excessive levels of androgens and ovulatory dysfunction, and is a common endocrine disorder in women of reproductive age. Polycystic ovary syndrome arises as a result of polygenic susceptibility in combination with environmental influences that might include epigenetic alterations and in utero programming. In addition to the well recognised clinical manifestations of hyperandrogenism and ovulatory dysfunction, women with polycystic ovary syndrome have an increased risk of adverse mental health outcomes, pregnancy complications, and cardiometabolic disease. Unlicensed treatments have limited efficacy, mostly because drug development has been hampered by an incomplete understanding of the underlying pathophysiological processes. Advances in genetics, metabolomics, and adipocyte biology have improved our understanding of key changes in neuroendocrine, enteroendocrine, and steroidogenic pathways, including increased gonadotrophin releasing hormone pulsatility, androgen excess, insulin resistance, and changes in the gut microbiome. Many patients with polycystic ovary syndrome have high levels of 11-oxygenated androgens, with high androgenic potency, that might mediate metabolic risk. These advances have prompted the development of new treatments, including those that target the neurokinin-kisspeptin axis upstream of gonadotrophin releasing hormone, with the potential to lessen adverse clinical sequelae and improve patient outcomes.
Keywords: Endocrinology; Medicine; Metabolic diseases; Obstetrics; Pharmacology; Reproductive medicine.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: We have read and understood the BMJ Policy on declaration of interests and declare the following interests: AR has undertaken educational activities funded by Pfizer and Diurnal, and received grant funding from the Waterloo Foundation. AR has participated as principal investigator in clinical trials funded by Neurocrine Biosciences, Sparrow Pharmaceuticals, Diurnal, and Ascendis.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
