The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: a Systematic Review
- PMID: 37859810
- PMCID: PMC10583582
- DOI: 10.7150/jca.87335
The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: a Systematic Review
Abstract
Purpose: Immune checkpoint inhibitor (ICI) use can lead to immune-related adverse events (irAEs) that require treatment with immunosuppressive medications in moderate to severe cases. Oncology society guidelines recommend systemic steroids and immunosuppressants such as infliximab and vedolizumab for the treatment of refractory cases. Limited information is available about the safety profile and potential adverse effects of these immunosuppressants. We have investigated the safety profile of multiple immunosuppressants which are used in the treatment of ICI-related irAEs. Methods: We performed a systematic review of studies reporting irAEs, from ICI use, and their medical management with immunosuppressants in adult cancer patients. We searched MEDLINE, EMBASE, Cochrane Library, and ClinicalTrials.gov from inception through September 1, 2022, using the following keywords or their equivalents: ICI, immunosuppressant, and irAE. We extracted observational studies and clinical trials that matched our criteria. A random effects model was used to estimate the overall incidence of infections associated with the treatment of irAEs. Results: Among the 11 studies included in this review (1036 total patients), melanoma (548 patients, 52.9%) was the most common primary cancer, followed by lung cancer (139 patients, 13.4%) and genitourinary cancers (131 patients, 12.6%). PD-1/PD-L1 monotherapy (460 patients, 44.4%) was used most, followed by a combination of PD-1/PD-L1 and CTLA-4 therapy (350 patients, 33.8%) and CTLA-4 monotherapy (226 patients, 22%). A total of 1024 (98.8%) patients had their irAEs treated with systemic steroids with majority having colitis and hepatobiliary irAEs; 335 patients (32.3%) were also treated with infliximab (mainly for colitis). Our review found 22.3% of patients treated for irAEs developed infectious adverse events (95% CI: 15.6%-29.1%, p<0.001). Among the 3 studies reporting the types of infections (41 total patients), bacterial (80.5%), followed by fungal (36.6%), infections were most common. Conclusions: Adverse events from irAE treatment occurred in about one-third of patients that received either steroids or a combination of steroids and other immunosuppressants. Clinicians should be aware of these immunosuppressant-related adverse effects, which can negatively impact cancer treatment and patient outcomes, when treating irAEs and consider shortening treatment duration or using alternative strategies when possible to mitigate these complications, future prospective studies should further investigate the safety of immunosuppressants in treating irAEs.
Keywords: complications; immune checkpoint inhibitor; immune-related adverse events; immunosuppressant; infection; meta-analysis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
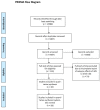

References
-
- Brown VT, Antol DD, Racsa PN, Ward MA, Naidoo J. Real-World Incidence and Management of Immune-Related Adverse Events from Immune Checkpoint Inhibitors: Retrospective Claims-Based Analysis. Cancer Invest. 2021;39(10):789–96. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

