Mifepristone inhibits hepatoma growth by enhancing the GR-HSP60-survivin interaction to facilitate survivin degradation
- PMID: 37859823
- PMCID: PMC10583585
- DOI: 10.7150/jca.86611
Mifepristone inhibits hepatoma growth by enhancing the GR-HSP60-survivin interaction to facilitate survivin degradation
Abstract
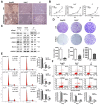
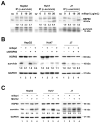
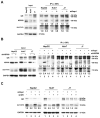
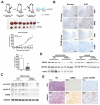
Silencing of heat shock protein 60 (HSP60) suppresses the growth of hepatocellular carcinoma (HCC). Mifepristone inhibits HSP60 mRNA expression in Chlamydophila-infected epithelial cells. The aim of this study was to determine whether mifepristone could inhibit the growth of HCC cells by affecting the functions of HSP60. The effect of mifepristone on cell viability was examined by flow cytometry and a cell proliferation assay. Protein-protein interactions were examined using the immunoprecipitation assay. The anti-tumor effect of mifepristone was evaluated using a xenograft model. Our results indicated that mifepristone induces cell cycle arrest at the G1 phase and early-stage apoptosis in HCC cells. Instead of reducing the total amount of HSP60, mifepristone induced the release of mitochondrial HSP60 into the cytosol by causing a loss of ΔΨm, thereby enhancing glucocorticoid receptor (GR)-HSP60-survivin complex formation as well as survivin degradation. Animal models have confirmed the growth inhibitory effects of mifepristone on HCC, including changes in the abundance of HSP60 in mitochondria and cytosol, decreased survivin and Ki-67-positive cells, as well as increased cell apoptosis. In conclusion, the inhibition of HCC growth by mifepristone may be achieved by altering the subcellular distribution of HSP60 to enhance the formation of cytosolic GR-HSP60-survivin complexes in the cells, leading to the degradation of survivin.
Keywords: HSP60; glucocorticoid receptor; hepatocellular carcinoma; mifepristone; survivin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Targeting HSP60 by subcutaneous injections of jetPEI/HSP60-shRNA destabilizes cytoplasmic survivin and inhibits hepatocellular carcinoma growth.Mol Carcinog. 2018 Sep;57(9):1087-1101. doi: 10.1002/mc.22827. Epub 2018 May 2. Mol Carcinog. 2018. PMID: 29672920
-
Functional Compartmentalization of HSP60-Survivin Interaction between Mitochondria and Cytosol in Cancer Cells.Cells. 2019 Dec 19;9(1):23. doi: 10.3390/cells9010023. Cells. 2019. PMID: 31861751 Free PMC article. Review.
-
PKCδ mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression.Free Radic Biol Med. 2021 Feb 1;163:69-87. doi: 10.1016/j.freeradbiomed.2020.12.003. Epub 2020 Dec 8. Free Radic Biol Med. 2021. PMID: 33307168
-
Mifepristone sensitizing cisplatin for cervical adenocarcinoma HeLa cell sensitivity to chemotherapy and its mechanism.Eur J Gynaecol Oncol. 2013;34(2):142-7. Eur J Gynaecol Oncol. 2013. PMID: 23781585
-
Survivin in survival of hepatocellular carcinoma.Cancer Lett. 2016 Sep 1;379(2):184-90. doi: 10.1016/j.canlet.2015.06.016. Epub 2015 Jun 25. Cancer Lett. 2016. PMID: 26118774 Review.
Cited by
-
Cetylpyridinium chloride inhibits hepatocellular carcinoma growth and metastasis through regulating epithelial-mesenchymal transition and apoptosis.PLoS One. 2024 Sep 20;19(9):e0310391. doi: 10.1371/journal.pone.0310391. eCollection 2024. PLoS One. 2024. PMID: 39302935 Free PMC article.
-
Molecular Chaperonin HSP60: Current Understanding and Future Prospects.Int J Mol Sci. 2024 May 17;25(10):5483. doi: 10.3390/ijms25105483. Int J Mol Sci. 2024. PMID: 38791521 Free PMC article. Review.
References
-
- Khalaf N, Ying J, Mittal S, Temple S, Kanwal F, Davila J. et al. Natural History of Untreated Hepatocellular Carcinoma in a US Cohort and the Role of Cancer Surveillance. Clin Gastroenterol Hepatol. 2017;15:273–81. e1. - PubMed
-
- Huang YH, Lin KH, Yu JS, Wu TJ, Lee WC, Chao CC. et al. Targeting HSP60 by subcutaneous injections of jetPEI/HSP60-shRNA destabilizes cytoplasmic survivin and inhibits hepatocellular carcinoma growth. Mol Carcinog. 2018;57:1087–101. - PubMed
-
- Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M. et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–5. - PubMed
-
- Ishida K, Yamazaki T, Motohashi K, Kobayashi M, Matsuo J, Osaki T. et al. Effect of the steroid receptor antagonist RU486 (mifepristone) on an IFNgamma-induced persistent Chlamydophila pneumoniae infection model in epithelial HEp-2 cells. J Infect Chemother. 2012;18:22–9. - PubMed
-
- Baulieu EE. RU 486 (mifepristone). A short overview of its mechanisms of action and clinical uses at the end of 1996. Ann N Y Acad Sci. 1997;828:47–58. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

