Levodopa-induced dyskinesia: interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation
- PMID: 37860013
- PMCID: PMC10582719
- DOI: 10.3389/fimmu.2023.1253273
Levodopa-induced dyskinesia: interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation
Abstract
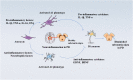
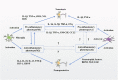
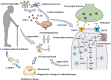
Parkinson's disease (PD) is a common neurodegenerative disorder of middle-aged and elderly people, clinically characterized by resting tremor, myotonia, reduced movement, and impaired postural balance. Clinically, patients with PD are often administered levodopa (L-DOPA) to improve their symptoms. However, after years of L-DOPA treatment, most patients experience complications of varying severity, including the "on-off phenomenon", decreased efficacy, and levodopa-induced dyskinesia (LID). The development of LID can seriously affect the quality of life of patients, but its pathogenesis is unclear and effective treatments are lacking. Glutamic acid (Glu)-mediated changes in synaptic plasticity play a major role in LID. The N-methyl-D-aspartic acid receptor (NMDAR), an ionotropic glutamate receptor, is closely associated with synaptic plasticity, and neuroinflammation can modulate NMDAR activation or expression; in addition, neuroinflammation may be involved in the development of LID. However, it is not clear whether NMDA receptors are co-regulated with neuroinflammation during LID formation. Here we review how neuroinflammation mediates the development of LID through the regulation of NMDA receptors, and assess whether common anti-inflammatory drugs and NMDA receptor antagonists may be able to mitigate the development of LID through the regulation of central neuroinflammation, thereby providing a new theoretical basis for finding new therapeutic targets for LID.
Keywords: N-methyl-D-aspartate receptor; Parkinson’s disease; levodopa; levodopa-induced dyskinesia; neuroinflammation.
Copyright © 2023 Zhang, Liu, Tuo, Zhang, Zhang, Yu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

