The novel cyclophilin inhibitor C105SR reduces hepatic ischaemia-reperfusion injury via mitoprotection
- PMID: 37860051
- PMCID: PMC10582583
- DOI: 10.1016/j.jhepr.2023.100876
The novel cyclophilin inhibitor C105SR reduces hepatic ischaemia-reperfusion injury via mitoprotection
Abstract
Background & aims: Mitochondrial permeability transition pore (mPTP) opening is critical for mediating cell death during hepatic ischaemia-reperfusion injury (IRI). Blocking mPTP opening by inhibiting cyclophilin D (CypD) is a promising pharmacological approach for the treatment of IRI. Here, we show that diastereoisomers of a new class of small-molecule cyclophilin inhibitors (SMCypIs) have properties that make them attractive candidates for the development of therapeutic agents against liver IRI.
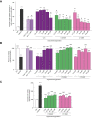
Methods: Derivatives of the parent SMCypI were synthesised and evaluated for their ability to inhibit CypD peptidyl-prolyl cis-trans isomerase (PPIase) activity and for their mitoprotective properties, evaluated by measuring mitochondrial swelling and calcium retention capacity in liver mitochondria. The ability of the selected compounds to inhibit mPTP opening was evaluated in cells subjected to hypoxia/reoxygenation using a calcein/cobalt assay. Their ability to inhibit cell death was evaluated in cells subjected to hypoxia/reoxygenation by measuring lactate dehydrogenase (LDH) release, propidium iodide staining, and cell viability. The compound performing best in vitro was selected for in vivo efficacy evaluation in a mouse model of hepatic IRI.
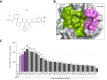
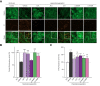
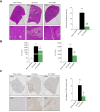
Results: The two compounds that showed the strongest inhibition of CypD PPIase activity and mPTP opening, C105 and C110, were selected. Their SR diastereoisomers carried the activity of the racemic mixture and exhibited mitoprotective properties superior to those of the known macrocyclic cyclophilin inhibitors cyclosporin A and alisporivir. C105SR was more potent than C110SR in inhibiting mPTP opening and prevented cell death in a model of hypoxia/reoxygenation. Finally, C105SR substantially protected against hepatic IRI in vivo by reducing hepatocyte necrosis and apoptosis.
Conclusions: We identified a novel cyclophilin inhibitor with strong mitoprotective properties both in vitro and in vivo that represents a promising candidate for cellular protection in hepatic IRI.
Impact and implications: Hepatic ischaemia-reperfusion injury (IRI) is one of the main causes of morbidity and mortality during or after liver surgery. However, no effective therapies are available to prevent or treat this devastating syndrome. An attractive strategy to prevent hepatic IRI aims at reducing cell death by targeting mitochondrial permeability transition pore opening, a phenomenon regulated by cyclophilin D. Here, we identified a new small-molecule cyclophilin inhibitor, and demonstrated the enhanced mitoprotective and hepatoprotective properties of one of its diastereoisomers both in vitro and in vivo, making it an attractive lead compound for subsequent clinical development.
Keywords: Cellular protection; Liver necrosis; Mitochondrial calcium retention capacity; Mitochondrial permeability transition pore; Mitochondrial swelling; Peptidyl-prolyl cis-trans isomerase activity.
© 2023 The Authors.
Conflict of interest statement
The authors have no conflict of interest to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Howard T.K., Klintmalm G.B., Cofer J.B., Husberg B.S., Goldstein R.M., Gonwa T.A. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. - PubMed
-
- Fellström B., Aküyrek L.M., Backman U., Larsson E., Melin J., Zezina L. Postischemic reperfusion injury and allograft arteriosclerosis. Transpl Proc. 1998;30:4278–4280. - PubMed
-
- Zhou J., Chen J., Wei Q., Saeb-Parsy K., Xu X. The role of ischaemia/reperfusion injury in early hepatic allograft dysfunction. Liver Transpl. 2020;26:1034–1048. - PubMed
-
- Halestrap A.P. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

