In vivo evaluation of the pharmacokinetic interactions between almonertinib and rivaroxaban, almonertinib and apixaban
- PMID: 37860116
- PMCID: PMC10582335
- DOI: 10.3389/fphar.2023.1263975
In vivo evaluation of the pharmacokinetic interactions between almonertinib and rivaroxaban, almonertinib and apixaban
Abstract
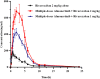
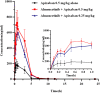
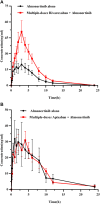
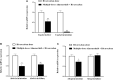
Background: Almonertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), is commonly used as a first-line treatment for non-small cell lung cancer (NSCLC) patients with EGFR T790M mutations. Rivaroxaban and apixaban are a selective, direct factor Xa inhibitor used to treat venous thromboembolism (VTE), which is a frequent complication of NSCLC. Rivaroxaban and apixaban are substrates of CYP3A4, P-gp and BCRP, whereas almonertinib is an inhibitor of P-gp and BCRP. Rivaroxaban or apixaban are often prescribed together with almonertinib in NSCLC patients, but clear information on pharmacokinetic drug interaction is lacking. Therefore, this study aimed to unravel the extent of interactions between almonertinib-rivaroxaban and almonertinib apixaban in rats, and whether the pharmacokinetic interaction can be mitigated by rivaroxaban and apixaban dose adjustment. Methods: Rats were divided into ten groups (n = 6) that received rivaroxaban (2 mg/kg) (group 1), apixaban (0.5 mg/kg) (group 2), almonertinib (15 mg/kg) (group 3, group 4), almonertinib with rivaroxaban (2 mg/kg) (group 5), almonertinib with rivaroxaban (1 mg/kg) (group 6), almonertinib with apixaban (0.5 mg/kg) (group 7), almonertinib with apixaban (0.25 mg/kg) (group 8), rivaroxaban (2 mg/kg) with almonertinib (group 9), apixaban (0.5 mg/kg) with almonertinib (group 10). The concentrations of drugs were determined by an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). The levels of messenger RNA were determined using quantitative real-time polymerase chain reaction (qRT-PCR). Results and Discussion: The results indicate that almonertinib increased the Cmax and AUC0-t of 2 mg/kg rivaroxaban by 3.30 and 3.60-fold, 1 mg/kg rivaroxaban by 1.28 and 1.90-fold. Almonertinib increased the Cmax and AUC0-t of 0.5 mg/kg apixaban by 2.69 and 2.87-fold, 0.25 mg/kg apixaban by 2.19 and 2.06-fold. In addition, rivaroxaban also increased systemic exposure to almonertinib. The results of qRT-PCR showed that almonertinib reduced the expression of Cyp3a1 in liver and intestine, and Abcb1a, Abcg2 in intestine and kidney. The pharmacokinetic results suggest that it is important to take special care of the interactions of these drugs in clinical applications.
Keywords: UPLC-MS/MS; almonertinib; apixaban; drug-drug interactions; pharmacokinetics; rivaroxaban.
Copyright © 2023 Wang, Li, He, Fu, Li, Zhou and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alsubaie N. S., Al R. S., Alshouimi R. A., Alzahrani M. Y., Al Y. M., Almutairi A. R., et al. (2021). The use of direct oral anticoagulants for thromboprophylaxis or treatment of cancer-associated venous thromboembolism: A meta-analysis and review of the guidelines. Thromb. J. 19 (1), 76. 10.1186/s12959-021-00326-2 - DOI - PMC - PubMed
-
- Chang S. H., Chou I. J., Yeh Y. H., Chiou M. J., Wen M. S., Kuo C. T., et al. (2017). Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 318 (13), 1250–1259. 10.1001/jama.2017.13883 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

