Evolution and implementation of radiographic response criteria in neuro-oncology
- PMID: 37860269
- PMCID: PMC10584081
- DOI: 10.1093/noajnl/vdad118
Evolution and implementation of radiographic response criteria in neuro-oncology
Abstract
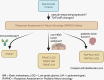
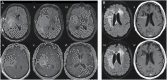
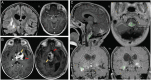
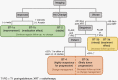
Radiographic response assessment in neuro-oncology is critical in clinical practice and trials. Conventional criteria, such as the MacDonald and response assessment in neuro-oncology (RANO) criteria, rely on bidimensional (2D) measurements of a single tumor cross-section. Although RANO criteria are established for response assessment in clinical trials, there is a critical need to address the complexity of brain tumor treatment response with multiple new approaches being proposed. These include volumetric analysis of tumor compartments, structured MRI reporting systems like the Brain Tumor Reporting and Data System, and standardized approaches to advanced imaging techniques to distinguish tumor response from treatment effects. In this review, we discuss the strengths and limitations of different neuro-oncology response criteria and summarize current research findings on the role of novel response methods in neuro-oncology clinical trials and practice.
Keywords: BT-RADS; RANO; RAPNO; Response Assessment; Volumetrics.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
M.S.A. has collaborations with Visage Imaging, Inc., Blue Earth Diagnostics, Telix, and AAA.. N.G. and P.L received honoraria for lectures from Blue Earth Diagnostics. N.G. received honoraria for advisory board participation from Telix Pharmaceuticals.
Figures
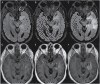
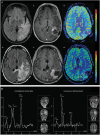
References
-
- Huang RY, Wen PY.. Response assessment in neuro-oncology criteria and clinical endpoints. Magn Reson Imaging Clin N Am. 2016;24(4):705–718. - PubMed
-
- Chinot OL, Wick W, Saran F, et al. . AVAglio: a phase III trial of bevacizumab added to standard radiotherapy and temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2011;29(suppl_15):TPS136.
-
- Gilbert MR, Dignam J, Won M, et al. . RTOG 0825: phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J Clin Oncol. 2013;31(suppl_18):1. - PubMed
-
- Nowosielski M, Wen PY.. Imaging criteria in neuro-oncology. Semin Neurol. 2018;38(1):24–31. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
