The highly and perpetually upregulated thyroglobulin gene is a hallmark of functional thyrocytes
- PMID: 37860816
- PMCID: PMC10582334
- DOI: 10.3389/fcell.2023.1265407
The highly and perpetually upregulated thyroglobulin gene is a hallmark of functional thyrocytes
Erratum in
-
Corrigendum: The highly and perpetually upregulated thyroglobulin gene is a hallmark of functional thyrocytes.Front Cell Dev Biol. 2025 Mar 10;13:1571466. doi: 10.3389/fcell.2025.1571466. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40129566 Free PMC article.
Abstract
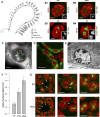
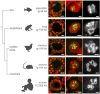
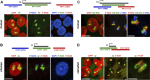
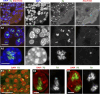
Abnormalities are indispensable for studying normal biological processes and mechanisms. In the present work, we draw attention to the remarkable phenomenon of a perpetually and robustly upregulated gene, the thyroglobulin gene (Tg). The gene is expressed in the thyroid gland and, as it has been recently demonstrated, forms so-called transcription loops, easily observable by light microscopy. Using this feature, we show that Tg is expressed at a high level from the moment a thyroid cell acquires its identity and both alleles remain highly active over the entire life of the cell, i.e., for months or years depending on the species. We demonstrate that this high upregulation is characteristic of thyroglobulin genes in all major vertebrate groups. We provide evidence that Tg is not influenced by the thyroid hormone status, does not oscillate round the clock and is expressed during both the exocrine and endocrine phases of thyrocyte activity. We conclude that the thyroglobulin gene represents a unique and valuable model to study the maintenance of a high transcriptional upregulation.
Keywords: gene upregulation; thyroglobulin gene; thyroid hormones; transcription; transcription loop.
Copyright © 2023 Ullrich, Leidescher, Feodorova, Thanisch, Fini, Kaspers, Weber, Markova, Führer, Romitti, Krebs, Blum, Leonhardt, Costagliola, Heuer and Solovei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

