Evinacumab for Pediatric Patients With Homozygous Familial Hypercholesterolemia
- PMID: 37860863
- PMCID: PMC10814999
- DOI: 10.1161/CIRCULATIONAHA.123.065529
Evinacumab for Pediatric Patients With Homozygous Familial Hypercholesterolemia
Erratum in
-
Correction to: Evinacumab for Pediatric Patients With Homozygous Familial Hypercholesterolemia.Circulation. 2025 Jul 8;152(1):e22. doi: 10.1161/CIR.0000000000001350. Epub 2025 Jul 7. Circulation. 2025. PMID: 40623081 Free PMC article. No abstract available.
Abstract
Background: Homozygous familial hypercholesterolemia (HoFH) is a rare genetic disorder characterized by severely elevated low-density lipoprotein cholesterol (LDL-C) levels due to profoundly defective LDL receptor (LDLR) function. Given that severely elevated LDL-C starts in utero, atherosclerosis often presents during childhood or adolescence, creating a largely unmet need for aggressive LDLR-independent lipid-lowering therapies in young patients with HoFH. Here we present the first evaluation of the efficacy and safety of evinacumab, a novel LDLR-independent lipid-lowering therapy, in pediatric patients with HoFH from parts A and B of a 3-part study.
Methods: The phase 3, part B, open-label study treated 14 patients 5 to 11 years of age with genetically proven HoFH (true homozygotes and compound heterozygotes) with LDL-C >130 mg/dL, despite optimized lipid-lowering therapy (including LDLR-independent apheresis and lomitapide), with intravenous evinacumab 15 mg/kg every 4 weeks.
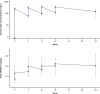
Results: Evinacumab treatment rapidly and durably (through week 24) decreased LDL-C with profound reduction in the first week, with a mean (SE) LDL-C reduction of -48.3% (10.4%) from baseline to week 24. ApoB (mean [SE], -41.3% [9.0%]), non-high-density lipoprotein cholesterol (-48.9% [9.8%]), and total cholesterol (-49.1% [8.1%]) were similarly decreased. Treatment-emergent adverse events were reported in 10 (71.4%) patients; however, only 2 (14.3%) reported events that were considered to be treatment-related (nausea and abdominal pain). One serious treatment-emergent adverse event of tonsillitis occurred (n=1), but this was not considered treatment-related.
Conclusions: Evinacumab constitutes a new treatment for pediatric patients with HoFH and inadequately controlled LDL-C despite optimized lipid-lowering therapy, lowering LDL-C levels by nearly half in these extremely high-risk and difficult-to-treat individuals.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04233918.
Keywords: angiopoietin-like protein 3; atherosclerosis; evinacumab; homozygous familial hypercholesterolemia; lipids; lipoprotein; pediatrics.
Conflict of interest statement
Figures


Comment in
-
LDL-C-Lowering Therapies for Adults and Children With Homozygous Familial Hypercholesterolemia: Challenges and Successes.Circulation. 2024 Jan 30;149(5):363-366. doi: 10.1161/CIRCULATIONAHA.123.067241. Epub 2024 Jan 29. Circulation. 2024. PMID: 38285739 No abstract available.
References
-
- Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, Bruckert E, Freiberger T, Gaudet D, Harada-Shiba M, et al. 2023 Update on European Atherosclerosis Society consensus statement on homozygous familial hypercholesterolaemia: new treatments and clinical guidance. Eur Heart J. 2023;44:2277–2291. doi: 10.1093/eurheartj/ehad197 - PMC - PubMed
-
- Tromp TR, Hartgers ML, Hovingh GK, Vallejo-Vaz AJ, Ray KK, Soran H, Freiberger T, Bertolini S, Harada-Shiba M, Blom DJ, et al. ; Homozygous Familial Hypercholesterolaemia International Clinical Collaborators. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet. 2022;399:719–728. doi: 10.1016/S0140-6736(21)02001-8 - PMC - PubMed
-
- Brown MS, Goldstein JL. Familial hypercholesterolemia: a genetic defect in the low-density lipoprotein receptor. N Engl J Med. 1976;294:1386–1390. doi: 10.1056/NEJM197606172942509 - PubMed
-
- Brown MS, Kovanen PT, Goldstein JL, Eeckels R, Vandenberghe K, van den Berghe H, Fryns JP, Cassiman JJ. Prenatal diagnosis of homozygous familial hypercholesterolaemia. Expression of a genetic receptor disease in utero. Lancet. 1978;1:526–529. doi: 10.1016/s0140-6736(78)90552-4 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

