Transferrin Receptor-Targeted Iduronate-2-sulfatase Penetrates the Blood-Retinal Barrier and Improves Retinopathy in Mucopolysaccharidosis II Mice
- PMID: 37860991
- PMCID: PMC10630942
- DOI: 10.1021/acs.molpharmaceut.3c00736
Transferrin Receptor-Targeted Iduronate-2-sulfatase Penetrates the Blood-Retinal Barrier and Improves Retinopathy in Mucopolysaccharidosis II Mice
Abstract
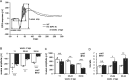
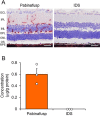
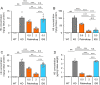
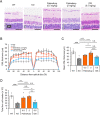
Mucopolysaccharidoses (MPSs) make up a group of lysosomal storage diseases characterized by the aberrant accumulation of glycosaminoglycans throughout the body. Patients with MPSs display various signs and symptoms, such as retinopathy, which is also observed in patients with MPS II. Unfortunately, retinal disorders in MPS II are resistant to conventional intravenous enzyme-replacement therapy because the blood-retinal barrier (BRB) impedes drug penetration. In this study, we show that a fusion protein, designated pabinafusp alfa, consisting of an antihuman transferrin receptor antibody and iduronate-2-sulfatase (IDS), crosses the BRB and reaches the retina in a murine model of MPS II. We found that retinal function, as assessed by electroretinography (ERG) in MPS II mice, deteriorated with age. Early intervention with repeated intravenous treatment of pabinafusp alfa decreased heparan sulfate deposition in the retina, optic nerve, and visual cortex, thus preserving or even improving the ERG response in MPS II mice. Histological analysis further revealed that pabinafusp alfa mitigated the loss of the photoreceptor layer observed in diseased mice. In contrast, recombinant nonfused IDS failed to reach the retina and hardly affected the retinal disease. These results support the hypothesis that transferrin receptor-targeted IDS can penetrate the BRB, thereby ameliorating retinal dysfunction in MPS II.
Keywords: blood-retinal barrier; electroretinography; mucopolysaccharidosis II; pabinafusp alfa; retinopathy; transferrin receptor.
Conflict of interest statement
The authors declare the following competing financial interest(s): A. Imakiire, H. Morimoto, H.S., T.M., E.Y., A. Inoue, H. Morioka, T.K., A.M., R.S. R.K., T.H., H.S., and K.M. are employees and/or stockholders of JCR Pharmaceuticals Co., Ltd.
Figures


References
-
- Neufeld E. F.; Muenzer J.. The mucopolysaccharidoses. In The Metabolic & Molecular Bases of Inherited Disease; Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Eds.; McGraw Hill: New York, USA, 2001; pp 3421–3452.
-
- Tylki-Szymańska A. Mucopolysaccharidosis type II, Hunter’s syndrome. Pediatr. Endocrinol. Rev. 2014, 12 (Suppl 1), 107–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

