Immune Activation Profiles Elicited by Distinct, Repeated TLR Agonist Infusions in Rhesus Macaques
- PMID: 37861342
- PMCID: PMC10656433
- DOI: 10.4049/jimmunol.2300424
Immune Activation Profiles Elicited by Distinct, Repeated TLR Agonist Infusions in Rhesus Macaques
Abstract
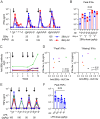
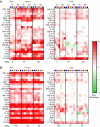
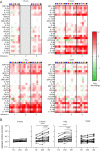
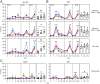
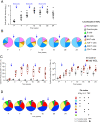
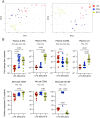
TLR agonists are a promising class of immune system stimulants investigated for immunomodulatory applications in cancer immunotherapy and viral diseases. In this study, we sought to characterize the safety and immune activation achieved by different TLR agonists in rhesus macaques (Macaca mulatta), a useful preclinical model of complex immune interactions. Macaques received one of three TLR agonists, followed by plasma cytokine, immune cell subset representation, and blood cell activation measurements. The TLR4 agonist LPS administered i.v. induced very transient immune activation, including TNF-α expression and monocyte activation. The TLR7/8 agonist 2BXy elicited more persistent cytokine expression, including type I IFN, IL-1RA, and the proinflammatory IL-6, along with T cell and monocyte activation. Delivery of 2BXy i.v. and i.m. achieved comparable immune activation, which increased with escalating dose. Finally, i.v. bacillus Calmette-Guérin (BCG) vaccination (which activates multiple TLRs, especially TLR2/4) elicited the most pronounced and persistent innate and adaptive immune response, including strong induction of IFN-γ, IL-6, and IL-1RA. Strikingly, monocyte, T cell, and NK cell expression of the proliferation marker Ki67 increased dramatically following BCG vaccination. This aligned with a large increase in total and BCG-specific cells measured in the lung. Principal component analysis of the combined cytokine expression and cellular activation responses separated animals by treatment group, indicating distinct immune activation profiles induced by each agent. In sum, we report safe, effective doses and routes of administration for three TLR agonists that exhibit discrete immunomodulatory properties in primates and may be leveraged in future immunotherapeutic strategies.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


Similar articles
-
Characterization of the Infant Immune System and the Influence and Immunogenicity of BCG Vaccination in Infant and Adult Rhesus Macaques.Front Immunol. 2021 Oct 11;12:754589. doi: 10.3389/fimmu.2021.754589. eCollection 2021. Front Immunol. 2021. PMID: 34707617 Free PMC article.
-
Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques.Front Immunol. 2022 Feb 9;12:801799. doi: 10.3389/fimmu.2021.801799. eCollection 2021. Front Immunol. 2022. PMID: 35222355 Free PMC article.
-
rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector.PLoS One. 2008;3(11):e3790. doi: 10.1371/journal.pone.0003790. Epub 2008 Nov 21. PLoS One. 2008. PMID: 19023426 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Natural and trained innate immunity against Mycobacterium tuberculosis.Immunobiology. 2020 May;225(3):151951. doi: 10.1016/j.imbio.2020.151951. Epub 2020 Apr 27. Immunobiology. 2020. PMID: 32423788 Review.
Cited by
-
Regulating Immune Responses Induced by PEGylated Messenger RNA-Lipid Nanoparticle Vaccine.Vaccines (Basel). 2024 Dec 27;13(1):14. doi: 10.3390/vaccines13010014. Vaccines (Basel). 2024. PMID: 39852793 Free PMC article. Review.
References
-
- Kawai, T., Akira S.. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11: 373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

