Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study
- PMID: 37861461
- PMCID: PMC10784743
- DOI: 10.1158/2159-8290.CD-23-0964
Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study
Abstract
Xaluritamig (AMG 509) is a six-transmembrane epithelial antigen of the prostate 1 (STEAP1)-targeted T-cell engager designed to facilitate lysis of STEAP1-expressing cancer cells, such as those in advanced prostate cancer. This first-in-human study reports monotherapy dose exploration for patients with metastatic castration-resistant prostate cancer (mCRPC), primarily taxane pretreated. Ninety-seven patients received ≥1 intravenous dose ranging from 0.001 to 2.0 mg weekly or every 2 weeks. MTD was identified as 1.5 mg i.v. weekly via a 3-step dose. The most common treatment-related adverse events were cytokine release syndrome (CRS; 72%), fatigue (45%), and myalgia (34%). CRS occurred primarily during cycle 1 and improved with premedication and step dosing. Prostate-specific antigen (PSA) and RECIST responses across cohorts were encouraging [49% PSA50; 24% objective response rate (ORR)], with greater frequency at target doses ≥0.75 mg (59% PSA50; 41% ORR). Xaluritamig is a novel immunotherapy for prostate cancer that has shown encouraging results supporting further development.
Significance: Xaluritamig demonstrated encouraging responses (PSA and RECIST) compared with historical established treatments for patients with late-line mCRPC. This study provides proof of concept for T-cell engagers as a potential treatment for prostate cancer, validates STEAP1 as a target, and supports further clinical investigation of xaluritamig in prostate cancer. See related commentary by Hage Chehade et al., p. 20. See related article by Nolan-Stevaux et al., p. 90. This article is featured in Selected Articles from This Issue, p. 5.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
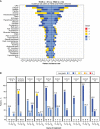

![Figure 3. Clinical activity of xaluritamig in evaluable patients. A, Best PSA percentage change from baseline. Asterisk indicates confirmed PSA responders, and dashed lines indicate PSA50 and PSA90 declines. B, Best percentage change in size of tumor target lesions. Dashed line indicates 30% reduction in tumor SLD from baseline. C, Example of patient showing response by PSA and radiographic assessments: CT scan and PSA curve over time of a heavily pretreated 65-year-old patient with stage IV prostate adenocarcinoma. Patient was enrolled into Cohort 11 (3-step 1.5 mg target dose of xaluritamig). CT scans showed three target lesions (two liver, one lymph node) and multiple nontarget lesions in the liver as well as two lymph nodes during screening. Patient achieved 99% PSA decline from baseline on C7D1 and PR (37.3% reduction of target lesions) after 2 cycles, which was confirmed at 16 weeks and maintained after 24 weeks. AEs occurred during C1 of treatment and included recurrent CRS, tinea faciei (both grade 1), rash, and worsening of back pain (both grade 2). During further treatment cycles, rash (grade 1), myalgia, and hyperkalemia (both grade 2) were reported. Patient remains on treatment at the time of publication. Red arrows indicate sites of tumor. D, Time on treatment for patients in high-dose cohorts. PSA and RECIST responses [RECIST evaluable (gray bars) and non–RECIST evaluable (white bars)] are presented for patients in high-dose cohorts. Patients whose treatment was ongoing are noted by an arrowhead. Double parallel lines (//) represent patients who have extended beyond 48 weeks: one patient is ongoing treatment at 90 weeks, one is ongoing treatment at 84 weeks, and one ended treatment at 58 weeks. NE, not evaluable; PD, progressive disease; SD, stable disease; SLD, sum of longest diameters.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/299b/10784743/cbc4437f6bd1/76fig3.gif)
Comment in
-
Bispecific T-cell Engagers in Metastatic Castration-Resistant Prostate Cancer.Cancer Discov. 2024 Jan 12;14(1):20-22. doi: 10.1158/2159-8290.CD-23-1230. Cancer Discov. 2024. PMID: 38213299
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- Cancer Fact Sheets, Prostate . Lyon (France), Geneva (Switzerland): International Agency for Research on Cancer, World Health Organization; 2020 [cited 2023 Jul 3]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet....
-
- Cancer Stat Facts: Prostate Cancer; [about 4 screens]. [cited 2023 Jul 3]. Available from: https://seer.cancer.gov/statfacts/html/prost.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

