The origins of nonideality exhibited by monoclonal antibodies and Fab fragments in human serum
- PMID: 37861473
- PMCID: PMC10659951
- DOI: 10.1002/pro.4812
The origins of nonideality exhibited by monoclonal antibodies and Fab fragments in human serum
Abstract
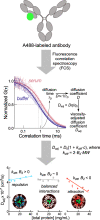
The development of therapeutic antibodies remains challenging, time-consuming, and expensive. A key contributing factor is a lack of understanding of how proteins are affected by complex biological environments such as serum and plasma. Nonideality due to attractive or repulsive interactions with cosolutes can alter the stability, aggregation propensity, and binding interactions of proteins in solution. Fluorescence correlation spectroscopy (FCS) can be used to measure apparent second virial coefficient (B2,app ) values for therapeutic and model monoclonal antibodies (mAbs) that capture the nature and strength of interactions with cosolutes directly in undiluted serum and similar complex biological media. Here, we use FCS-derived B2,app measurements to identify the components of human serum responsible for nonideal interactions with mAbs and Fab fragments. Most mAbs exhibit neutral or slightly attractive interactions with intact serum. Generally, mAbs display repulsive interactions with albumin and mildly attractive interactions with IgGs in the context of whole serum. Crucially, however, these attractive interactions are much stronger with pooled IgGs isolated from other serum components, indicating that the effects of serum nonideality can only be understood by studying the intact medium (rather than isolated components). Moreover, Fab fragments universally exhibited more attractive interactions than their parental mAbs, potentially rendering them more susceptible to nonideality-driven perturbations. FCS-based B2,app measurements have the potential to advance our understanding of how physiological environments impact protein-based therapeutics in general. Furthermore, incorporating such assays into preclinical biologics development may help de-risk molecules and make for a faster and more efficient development process.
Keywords: fluorescence correlation spectroscopy; macromolecular crowding; nonideality; second virial coefficient; therapeutic proteins.
© 2023 The Protein Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Abdollahpour‐Alitappeh M, Lotfinia M, Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, et al. Antibody–drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol. 2019;234:5628–5642. - PubMed
-
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, et al. The human plasma proteome. Mol Cell Proteomics. 2004;3:311–326. - PubMed
-
- Baek Y, Zydney AL. Intermolecular interactions in highly concentrated formulations of recombinant therapeutic proteins. Curr Opin Biotechnol. 2018;53:59–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

